JEE Main 2025 January 24 Chemistry Question Paper (Available): Answer key solutions PDF download
JEE Main 24 January 2025 Chemistry question paper along with the unofficial answer key solutions PDF can be checked here for both Shift 1 and 2.

JEE Main 2025 January 24 Chemistry Question Paper: For 24 January 2025, the JEE Main Chemistry Question Paper 2025 with Answer Key Solutions has been provided below for all the aspirants. Candidates who have appeared for the exam can check and download the JEE Main Chemistry Question Paper for the exam conducted on 24 January. The Chemistry section of the JEE Main exam comprises 25 questions, including multiple-choice and numerical-based questions. Students who have appeared for the exam can use the answer key to cross-check their responses and estimate their scores. Additionally, those preparing for upcoming shifts or sessions can use these resources to identify the pattern of questions, difficulty level, and frequently tested topics.
| Toughest Shift of JEE Main January 2025 (Session 1) | Easiest Shift of JEE Main January 2025 (Session 1) |
JEE Main 2025 January 24 Shift 2 Chemistry Question Paper with Answer Key Solutions
Below we have provided JEE Main 24 January 2025 Shift 2 Chemistry question paper with answer key solutions:
| Q.No. | Questions | Answers |
| 1 | Consider the following reaction S(s) + 3/2O2(g) → SO3(g) + 2x KJ SO2(g) + 1/2O2(g) →SO3(g) + y KJ Calculate ΔHr for the following reaction (KJ) S(s) + O2 → SO2(g) | y - 2x |
| 2 | The conditions and consequences that favour the t32ge1g configuration in a metal complex are | Weak field ligand; High spin complex |
| 3 | When ethane-1, 2-diammine is progressively added to aqueous solution of Nickel (II) chloride the sequence of colour change observed will be: | Green→Pale Blue→Blue→Violet |
| 4 | Statement 1: The first ionisation energy of Pb is greater than that of Sn. Statement 2: The first ionisation energy of Ge is greater than that of Si. | Statement 1 is correct but Statement 2 is incorrect. |
| 5 | 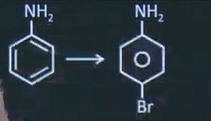 Above conversion can be done by using which reagents among the following. | Ac2O, Fe/Br2, H2O/H+ |
| 6 | Match the column: A. SC3+ - (P) 2.84 B. Ti2+ - (Q) 0 C. V2+ - (R) 5.92 D. Mn2+ - (S) 3.87 | A→(Q), B → (P), C→ (S), D → (R) |
| 7 | In a compound contains 54.2% carbon, 9.2% of hydrogen and the rest are oxygen. What is molecular formula of compound, if molecular mass is 132 g/mol? | C6H12O3 |
| 8 | Match the following nitrogenous bases present in List-l with their structures present in List-II. | A-(ii), B-(i), C-(iv), D-(iii) |
| 9 | Consider the following gaseous reaction H2(g) + I2(g)→2HI The above reaction is started with 'a' moles of H2 and 'b' moles of I2 in a closed container at a certain temperature T(K) till the equilibrium is established. Which one of the following plots correctly describes the progress of reaction? | |
| 10 | In the given compound no. of Sp and Sp2 hybridised carbon are | 3 and 3 |
| 11 | How many stereoisomers are possible for 5-Phenylpent-4-en-2-ol? | 4 |
| 12 | A hydrocarbon X which has molar mass 80g contains 90% carbon. Find degree of unsaturation in X. | 3 |
| 13 | The successive ionisation energy (IE) of an element 'X' is given Data given in KJ/mol. Find out the group number of element x. | Group-2 |
| 14 | Consider the following statements : Statement-l: Oxygen-oxygen bond in O3 is greater than O2. Statement-II: O-O bond order in O3 is 1.5 and O-O bond order in O2 is 2. | Both Statement 1 and Statement 2 are correct |
| 15 | In Carius method of estimation of halogen, 0.25 g of an organic compound gave 0.16 g of AgBr. What is the percentage of bromine in the compound (Given molar mass of Ag = 108, Br = 80) | 27% |
| 16 | Let k1, k2 and k3 be the rate constant of reaction and k = √k1k3/k2. Then find activation energy of overall reaction. (Given: Ea1 = 10 kJ/mol, Ea2 = 30 kJ/mol, Ea3 = 60 kJ/mol) | 20 |
JEE Main 24 January 2025 Question Paper with Answer Key Shift 1
Below we have provided the JEE Main 24 January 2025 Chemistry question paper with answer key solutions for Shift 1.
| Q.No. | Questions | Answers |
| 1 | Which of the following is the strongest oxidising agent? | Ce4+ |
| 2 | The difference in melting point and boiling point of oxygen and sulphur can be explained by | Atomicity |
| 3 | Ribose present in DNA is (A) It is a pentose sugar (B) Present in pyranose form (C) α anomeric carbon is present (D) Present in D configuration (E) It is reducing sugar in free form Choose the correct statements. | (A), (D) & (E) only |
| 4 | If the Ksp of Cr(OH)3 is 1.6 * 10-30 M4. The molar solubility of salt in water is 1.56 * 10-x, then value of x is | 8 |
| 5 | Find the most stable carbocation among the following carbocations. | |
| 6 | Which of the following is most reactive towards nucleophilic addition reaction? | Para-nitro benzaldehyde |
| 7 | In H2O, NH3 and CH4 (A) All central atoms are sp3 hybridised (B) Order of dipole moment is CH4 < NH3 < H2O (C) NH3 in H2O is basic in nature, NH3 and H2O are Bronsted-Lowry acid and bases respectively (D) Bond angle of H2O, NH3 and CH, respectively are 104.5°, 107° and 109.5° | A, B and D only |
| 8 | Which of the following is most reactive towards aq. HBr? | (P) |
| 9 | At the freezing point of water, the process is non-spontaneous, at boiling point it becomes spontaneous (Temperature varies linearly with pressure). The correct option is | ∆H = +ve ∆S = +ve |
| 10 | In the preparation of potassium permanganate from pyrolusite ore (MnO2), the fusion of the pyrolusite ore is done with an alkali metal hydroxide like KOH in the presence of air or an oxidising agent like KNO3, which first produces. | K2MnO4 |
| 11 | Statement 1: Duma's method is used for estimation of nitrogen Statement 2: In Duma's method N present in compound is converted to (NH4)2SO4 | Statement 1 is correct but Statement 2 is incorrect |
| 12 | Fe2+ + Ag+ → Fe3+ + Ag; Enet0______? Ag+ + e- → Ag; E° = x Fe2+ + 2e- → Fe; E° = y Fe3+ + 3e- → Fe; E° = z The value of Enet0 = ? | x + 2y - 3z |
| 13 | Consider the given reactions and choose proper solvent. | Statement-l: polar aprotic, Statement-II: polar aprotic |
| 14 | 2.32 x 103 kg of Fe3O4 reacts with 2.8 × 103 kg of CO according to the following reaction: Fe3O4 + CO → CO2 + Fe If x kg of Fe is formed. Find the value of x? | 1680 kg |
| 15 | Consider the following reaction | |
| 16 | When x g of Benzoic acid reacts with NaHCO3, 11.2 L of CO2 is released at 273 K and 1 atm pressure, calculate mass of benzoic acid in gram? | 61 gm |
| 17 | How many of the following cation shows characteristic coloured ppt. with K4[Fe(CN)6]? Cu2+, Ca2+, Ba2+, Fe3+, Zn2+, Mg2+, Mn2+ | 3 |
| 18 | Consider the following reaction of a complex compound The formula of complex is | [Co(NH3)5Cl] Cl2 |
| 19 | Consider the following plots of vapour pressure of a solution containing non-volatile solute versus temperature in K and choose the correct graph which represents depression in freezing of solvent. | |
| 20 | A student synthesised the compound given below By using one of the following compounds available in the lab and using following reagants: | |
| 21 | Select the incorrect statement about the modern periodic table. | Physical and chemical properties of elements are based on their atomic weight |
Also Read | JEE Main 24 Jan 2025 vs 30 Jan 2024 Shift Analysis: Hardest vs Easiest
JEE Main 24 January 2025 Chemistry Question Paper PDF Download
Download here JEE Main 24 Jan 2025 Chemistry question paper and answer key by CollegeDekho as provided above in PDF format:
| Shift | Chemistry Question Paper with Answers Links |
| Shift 1 | JEE Main 2025 Question Paper Chemistry 24 January Shift 1 PDF |
| Shift 2 | JEE Main 2025 Question Paper Chemistry 24 January Shift 2 PDF |
JEE Main 24 January 2025 Question Paper: Physics and Mathematics
For other subjects across both shifts, check and download JEE Main 24 January 2025 question paper here:
| Parameter | Question Paper Link |
| Physics | JEE Main 2025 January 24 Physics Question Paper |
| Mathematics | JEE Main 2025 January 24 Mathematics Question Paper |
JEE Main 24 January 2025 Chemistry Question Paper PDFs by Coaching Institutions
For both Shift 1 and Shift 2, JEE Main 24 January 2025 Chemistry answer key and question paper PDF links have been provided as released by Resonance, Aakash BYJU's, and Vedantu in the below table:
| Coaching Institute | Shifts | Chemistry Question Paper with Answer Key PDFs |
| Resonance | Shift 1 | Resonance JEE Main Chemistry Question Paper and Answer Key 2025 January 24 Shift 1 |
| Shift 2 | Resonance JEE Main Chemistry Question Paper and Answer Key 2025 January 24 Shift 2 | |
| Aakash | Shift 1 | Aakash JEE Main Chemistry Question Paper and Answer Key 2025 January 24 Shift 1 |
| Shift 2 | Aakash JEE Main Chemistry Question Paper and Answer Key 2025 January 24 Shift 2 | |
| Vedantu | Shift 1 | Vedantu JEE Main Chemistry Question Paper and Answer Key 2025 January 24 Shift 1 |
| Shift 2 | Vedantu JEE Main Chemistry Question Paper and Answer Key 2025 January 24 Shift 2 |
You may also find important |
| Parameter | Link |
| Safe Score | Expected Safe Score in JEE Mains January 2025 |
| Safe Percentile | Expected Safe Percentile in JEE Main 2025 Session 1 |
| Question Paper (All Days) | JEE Main Question Paper January 2025 Shift 1 and 2 |
| Answer Key (All Days) | JEE Main Answer Key Solutions January 2025 |
| JEE Main Percentile to Marks | Expected Marks for JEE Main Percentile January 2025 |
| Marks to Percentile | JEE Main Expected Percentile Score January 2025 |
| Cutoff Percentile | JEE Main Expected Cutoff Percentile January 2025 |
| Cutoff Marks | Minimum Marks in JEE Mains to Qualify for JEE Advanced 2025 |
Subject-wise JEE Mains Marks vs Percentile 2025 |
| Name of the Subject | Link |
| Physics | JEE Main Physics Expected Marks vs Percentile 2025 |
| Chemistry | JEE Main Expected Chemistry Marks vs Percentile 2025 |
| Mathematics | JEE Main Mathematics Expected Marks vs Percentile 2025 |
Keep visiting CollegeDekho for the latest Education News on entrance exams, board exams and admissions. You can also write to us at our email ID news@collegedekho.com.
