JEE Main 22 January 2025 Chemistry Question Paper with Answer Key Solutions (Available)
Download here the memory-based JEE Main 22 January 2025 Chemistry question paper with answer key solutions for both Shift 1 and Shift 2. Chemistry question paper PDF by top coaching institutes will also be available here.

JEE Main 22 January 2025 Chemistry Question Paper: Candidates can check out the unofficial JEE Main 22 January 2025 Chemistry question paper with answer key solutions on the page below. The answer keys have been provided for Shift 1 and 2. The question paper (memory-based questions) for Day 1 Chemistry were collected from the candidates, the answers were formed by our subject experts and provided on the page below. Additionally, the answers by other coaching institutes have also been provided here. Through this, candidates can evaluate their performance on the test. In case of doubt regarding a specific answer, candidates can cross-check with the other answer keys for clarity.
| Toughest Shift of JEE Main January 2025 (Session 1) | Easiest Shift of JEE Main January 2025 (Session 1) |
JEE Main 22 January 2025 Chemistry Shift 1 Question Paper with Answer Key Solutions
Here is JEE Main 22 January 2025 Chemistry question paper with answer key solutions for Shift 1:
| Q. No. | Questions | Answers |
| 1 | For [NiCL4]2- what is the charge on metal and shape of complex respectively? | +2, Tetrahedral |
| 2 | Compare boiling point of given solutions- (i) 10-4 NaCl (ii) 10-3 NaCl (iii) 10-2 NaCl (iv) 10-4 Urea | (iii)>(ii)>(i)>(iv) |
| 3 | The correct decreasing order of electronegativity is | F>Cl>Br>I |
| 4 | C6H6-NO2 reacts with (i) Sn/HCl, (ii) NaNO2/H4, (iii) CuCl/H4, and (iv) Na/dryether. Find the molecular weight of A. | 154 gm/mole |
| 5 | Which of the following has the maximum size out of Al3+, Mg2+, F-, Na+? | F- |
| 6 | How many compounds have linear shape SO2, BeCl2, N3-, I3-, NO+2, NO2? | 4 |
| 7 | CO2(g) + C(s) → 2CO(g) If initial pressure of CO2 is 0.6 atm and after equilibrium is established, total pressure is 0.8 atm. Then, find Kp. | 0.4 atm |
| 8 | Calculate Number of stereoisomers of 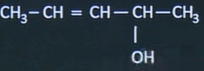 | 4 |
| 9 | Which of the following acids is also known as Vitamin C? | Ascorbic Acid |
| 10 | An electron of He+ is present in 3rd excited state. Find its de-Broglie wavelength. | 6.64λ |
| 11 | Which of the following lanthanide ion as 7e- in the outermost shell? | Gd+3 or EU+2 |
| 12 | 4f7 configuration is possible for: (a) Eu3+, (b) Eu2+, (c) Gd3+, (d) Tb3+, (e) Sm2+ | (b) and (c) |
| 13 | Given: NH2COONH4(s) → 2NH3(g) + CO2(g) If the partial pressure of CO2 gas at equilibrium is 0.4 atm and the total pressure is 1 atm, then the value of Kp at the same temperature is | 0.144 atm3 |
| 14 | CO2 gas is taken at 1 atm, 273K. Now it is allowed to pass through 0.1 M Ca/(OH)2 aq. Solution. Excess amount of Ca(OH)2 is neutralised with 40 mL of 0.1 M HCl. Then find volume of Ca(OH)2 initial taken if half of the amount Ca(OH)2 is reacted with CO2. | 40 mL |
| 15 | In a closed insulated container, a liquid is stirred with a paddle to increase the temperature, which of the following is true? | ΔE=w≠0, q=0 |
| 16 | Match the column and choose the correct option- (A) Electronegativity - (1) B<C<N<O (B) Cationic Size - (2)Li>Mg>Be (C) Metallic Character - (3) K>Mg>Al (D) Electron Affinity - (4) Cl<F<Br<I | A-1, B-2, C-3, D-4 |
| 17 | In Carius method 180 mg of organic compound gives 143.5 mg of AgCl. Find the percentage of Cl in the organic compound. (Nearest integer) | 20 |
| 18 | Two ampere current is allowed to pass through molten AlCl3 for 30 min. Find the mass of aluminium deposited in mg at cathode. (Nearest integer) | 336 mg |
| 19 | IUPAC name of | 2-methyl-3-methoxycarbonyl butanoic acid |
| 20 | Compare splitting energy for (i) K4[Fe(CN)6] (ii) [Cu(NH3)4]+2 (iii) K4[Fe(SCN)6] (iv) [Fe(en)3]Cl3 | (ii)>(i)>(iv)>(iii) |
Also Read | JEE Main 22 Jan 2025 vs 27 Jan 2024 Shift Analysis: Hardest vs Easiest
JEE Main 22 January 2025 Chemistry Shift 2 Question Paper with Answer Key Solutions
Here is JEE Main 22 January 2025 Chemistry question paper with answer key solutions for Shift 2.
| Q. No. | Questions | Answers |
| 1 | Density of 3 M NaOH is 1.25 g/ml. Molality of solutions is | 2.65 |
| 2 | Arrange according to CFSE. (i) [CO(NH3)4]2+ (ii) [CO(NH3)6]3+ (iii) [CO(NH3)6]2+ (iv) [CO(en)3]3+ | (iv)>(ii)>(iii)>(i) |
| 3 | The Product is | |
| 4 | What is correct order of stability of carbocation? | a>c>d>b |
| 5 | Which of the following anions will not undergoes disproportionate? | ClO-4 |
| 6 | Compare the dipole moment of (I) NF3 (II) CHCl3 (III) H2S (IV) HBr | II>III>IV>I |
| 7 | Calculate the radius of first excited state of He+ ion (in ????) | 1.058 |
| 8 | Given below are two statements- S-I: Lassaigne test is used for detection of Nitrogen, phosphorous, sulphur and Halogens. S-II: Lassaigne extract is made with magnesium metal. | Statement 1 is correct but Statement 2 is incorrect |
| 9 | Which one has two secondary Hydrogen atoms? (I) 2, 2, 4, 4-tetramethylheptane (II) 2, 2, 3, 4-tetramethylheptane (III) 2, 2, 3, 3-tetramethyloctane (IV) 3-ethyl-2, 2-dimethylpentane | 2, 2, 3, 4-tetramethylheptane |
| 10 | 200 mL of 0.2 M solution of NaOH is mixed with 400 mL of 0.5 M NaOH solution. Molarity of mixture is | 0.4 |
| 11 | In Ru and Nb, if in Ru, 4d electrons are x and in Nb, 4d electrons are y then find the sum of x and y. | 11 |
| 12 | Ni2+ + 2DMG → Complex How many hydrogen bonds are present in molecule of the complex? | 2 |
| 13 | How many R - Br can form isopentane? | 4 |
| 14 | Correct structure of L-Glyceraldehyde is | |
| 15 | Identify the extensive and intensive property? (I) Mass, volume, conductivity - Intensive property (II) Mass, temperature, heat, volume - Extensive property (III) Mass, volume, internal energy - Extensive property (IV) Density, temperature, moles, internal energy - Intensive property | Mass, volume, internal energy - Extensive property |
| 16 | Among Group-15 elements, what is the maximum covalency of an element having weakest E-E bond (E=element) | 5 |
| 17 | Secondary butyl cyclohexanol when reacts with Br2 in presence of sunlight produces | |
| 18 | What is the relation between Ksp and S of Zr3 (PO4)4 | S = [Ksp/6912]1/7 |
| 19 | Consider the following statements Statement-1 and Statement-2 and choose the correct option. Statement-1: During corrosion pure metal acts as anode and impure metal acts as cathode. Statement-2: Rate of corrosion is more in alkaline medium than in acidic medium. | Both Statement 1 and Statement 2 are incorrect |
Latest | JEE Main Question Paper 24 January 2025 Live Updates
JEE Main 22 January 2025 Chemistry Question Paper PDF Download
Download here JEE Main 22 Jan 2025 Chemistry question paper and answer key by CollegeDekho as provided above in PDF format:
| Shift | Chemistry Question Paper with Answers Links |
| Shift 1 | JEE Main 2025 Question Paper Chemistry 22 January Shift 1 PDF |
| Shift 2 | JEE Main 2025 Question Paper Chemistry 22 January Shift 2 PDF |
| Physics | JEE Main 22 Jan Physics Topic vs No. of Questions Analysis 2025 |
| Chemistry | JEE Main 22 Jan Chemistry Topic vs No. of Questions Analysis 2025 |
| Mathematics | JEE Main 22 Jan Mathematics Topic vs No. of Questions Analysis 2025 |
JEE Main 22 January 2025 Question Paper: Physics and Mathematics
For other subjects across both shifts, check and download JEE Main 22 January 2025 question paper here:
| Parameter | Question Paper Link |
| Physics | JEE Main 22 January 2025 Physics Question Paper with Answer Key Solutions |
| Mathematics | JEE Main 22 January 2025 Mathematics Question Paper with Answer Key Solutions |
JEE Main 22 January 2025 Chemistry Question Paper PDFs by Coaching Institutions
For both Shift 1 and Shift 2, JEE Main 22 January 2025 Chemistry answer key and question paper PDF links have been provided as released by Resonance, Aakash BYJU's, and Vedantu in the below table:
| Coaching Institute | Shifts | Chemistry Question Paper with Answer Key PDFs |
| Resonance | Shift 1 | PDF download link to be updated soon |
| Shift 2 | Resonance JEE Main Chemistry Question Paper and Answer Key 2025 January 22 | |
| Aakash | Shift 1 | Aakash JEE Main Chemistry Question Paper and Answer Key 2025 January 22 Shift 1 |
| Shift 2 | Aakash JEE Main Chemistry Question Paper and Answer Key 2025 January 22 Shift 2 | |
| Vedantu | Shift 1 | Vedantu JEE Main Chemistry Question Paper and Answer Key 2025 January 22 Shift 1 |
| Shift 2 | Vedantu JEE Main Chemistry Question Paper and Answer Key 2025 January 22 Shift 2 | |
| Narayana | Shift 1 | Narayana JEE Main Chemistry Question Paper and Answer Key 2025 January 22 Shift 1 |
You may also find important |
| Parameter | Link |
| Safe Score | Expected Safe Score in JEE Mains January 2025 |
| Safe Percentile | Expected Safe Percentile in JEE Main 2025 Session 1 |
| Question Paper (All Days) | JEE Main Question Paper January 2025 Shift 1 and 2 |
| Answer Key (All Days) | JEE Main Answer Key Solutions January 2025 |
| Exam Analysis (All Days) | JEE Main 2025 Session 1 Exam Analysis |
| JEE Main Percentile to Marks | Expected Marks for JEE Main Percentile January 2025 |
| Marks to Percentile | JEE Main Expected Percentile Score January 2025 |
| Cutoff Percentile | JEE Main Expected Cutoff Percentile January 2025 |
| Cutoff Marks | Minimum Marks in JEE Mains to Qualify for JEE Advanced 2025 |
Subject-wise JEE Mains Marks vs Percentile 2025 |
| Name of the Subject | Link |
| Physics | JEE Main Physics Expected Marks vs Percentile 2025 |
| Chemistry | JEE Main Chemistry Expected Marks vs Percentile 2025 |
| Mathematics | JEE Main Mathematics Expected Marks vs Percentile 2025 |
Keep visiting CollegeDekho for the latest Education News on entrance exams, board exams and admissions. You can also write to us at our email ID news@collegedekho.com.
