JEE Main 28 January 2025 Chemistry Question Paper with Answer Key Solutions (Available)
Download here the memory-based JEE Main 28 January 2025 Chemistry question paper with answer key solutions for both Shift 1 and Shift 2. Chemistry question paper PDF by top coaching institutes will also be available here.

JEE Main 28 January 2025 Chemistry Question Paper: The unofficial JEE Main Chemistry question paper with answer keys for January 28, 2025, is available to candidates on the website below. The solution keys were available following the conclusion of Shifts 1 and 2. Shifts 1 and 2 end edat 12 and 3 PM, respectively. Candidates will be asked to complete the question paper, and our subject matter experts will create the answers, which will be posted on the website below. Here, you will also see the responses from various coaching centers. Candidates can assess their test-taking performance in this way. Candidates can double-check their answers with the other answer keys if unsure about a particular response.
| Toughest Shift of JEE Main January 2025 (Session 1) | Easiest Shift of JEE Main January 2025 (Session 1) |
JEE Main 28 January 2025 Chemistry Shift 1 Question Paper with Answer Key Solutions
Check the JEE Main 28 January 2025 Chemistry question paper with answer key solutions for Shift 1 here:
| Q.No. | Questions | Answers |
|---|---|---|
| 1 | What is the rate of reaction for releasing CO2(g) with aq. NaHCO3 among the following?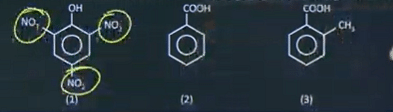 | 1>3>2 |
| 2 | 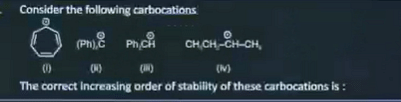 | i> ii> iii> iv |
| 3 | 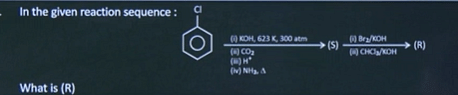 | 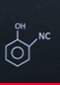 |
| 4 | Which of the following pair have square pyramidal shape? | BrF5, XeOF4 |
| 5 | Which of the following set of quantum numbers have same energy? | c,d |
| 6 | Δ1H of H(g) is 222kJ/ mol, Δ1H of O(g) is 250kJ/ mol, Δ1H of H2O is -248kJ/ mol. what is the value of Bond Energy of the O-H bond in H2O in kJ/ mol. | 471 |
| 7 | 70% of mass solution of HNO3 is taken heavy density 1.41 gm/ml. Calculate morality (Rounded off to nearest integer) | 16 |
| 8 | CH3CH2CH2NO2 & CH3CH2CH2NC | |
| 10 | A(Q), B(R), C(P), D(S) | |
| 11 | Among the following the incorrect order of atomic radius is | B> Al> Mg> F |
| 12 | 1g of a non electrolyte solute (MW=256g/mol) dissolved in 50g of solvent, freezing point of solution lowered by 0.40K. Calculate the molal depression constant of solvent. | 5 |
| 13 | A compound contains 14.4% Carbon, 1.2% Hydrogen, and 84.4% Chlorine. Calculate empirical formula mass of compound. (Molar mass of C=12, H=1, Cl=35.5) | 84 |
| 14 | ||
| 15 | Statement I is incorrect and Statement II is correct | |
| 16 | Which of the following has same energy in absence of electric and magnetic field for hydrogen atom | 2s, 2p |
| 17 | Which of the following reactions /tests can be used to distinguish acetaldehyde and acetone | D and E only |
| 18 | Which of the following give violet color in Borax bead test? | Mn2+ |
| 19 | Which of the following compounds have the same number of lone pair on central atomas CIF3 | XeF-5 |
| 20 | Statement 1: For Titration of oxalic acid using KMnO4, warming of acid solution is required whereas in case of Ferrous Ammonium Sulphate, it is done at room temperature. Statement 2: Fe2+ converts to Fe3+ ions during titration | Statement 1 and Statement 2 are correct |
JEE Main 28 January 2025 Chemistry Shift 2 Question Paper with Answer Key Solutions
Candidates shall obtain the JEE Main 28 January 2025 Chemistry Shift 2 question paper with answer key solutions here:
Q.No. | Questions | Answers |
1 | Consider the following oxides: V2O3 , V2O4, V2O5 Change in oxidation state of vanadium when amphoteric oxide reacts with acids to form VO4+ | 2 |
2 | Which has maximum oxidising power among the following | MnO4- |
3 | Number of paramagnetic species among the following is O2, O2+, O2- NO2 , NO, CO | 5 |
4 | How many of the following molecules are polar? | 5 |
5 | Which of the following complex is paramagnetic | {NlCl4}2- |
| 6 | 30 gm of HNO3 is added to a solution to prepare 75% w/w solution having density 1.25 g/mL. Volume of solution is | 32 mL |
| 7 | Both S-Iand S-II statements are correct | |
| 8 | For an elementary reaction A + B → C + D When volume becomes 1/3rd, rate of reaction becomes | 9 times |
| 9 | A (i), B (ii), C (iii), D (iv) | |
| 10 | The correct name of I & II in the following process is: | I- Sublimation II-Deposition |
| 11 | ||
| 12 | Which of the following biomolecules doesn't contain C1- C4 glycosidic linkage | Sucrose |
| 13 | Consider the following statements Statement I: In law of octaves, elements were arranged in ascending order of their atomic numbers. Statement II: Lothar Meyer, plotted the physical properties against atomic weight. Choose the correct answer from the options given below. | Both Statements I and II are incorrect |
| 14 | 'C' can give Tollen's test | |
| 15 | Consider the following oxides V2O3 , V2O4, V2O5 Oxidation state of vanadium in amphoteric oxide is | +5 |
| 16 | The bacterial life grows as per 1st order of kinetics.Which of the following graph is correct between N/N0 and t? | |
| 17 | How many of the following will give Benzoic acid on reaction with hot alkaline KMnO4? | 5 |
| 18 | By passing current in 600 mL of NaCl solution pH increase to 12. Find current (i) if electrolysis occurs for 10 min/assume 100% efficiency | 0.965 |
Also Read| Expected Important Topics for JEE Main 29 January 2025
JEE Main 28 January 2025 Chemistry Question Paper PDF Download
Download here JEE Main 28 Jan 2025 Chemistry question paper and answer key provided above in PDF format:
| Shift | Chemistry Question Paper with Answers Links |
| Shift 1 | JEE Main 28 Jan 2025 Chemistry Shift 1 Question Paper |
| Shift 2 | JEE Main 28 Jan 2025 Chemistry Shift 2 Question Paper |
JEE Main 28 January 2025 Question Paper: Physics and Mathematics
To access the JEE Main 28 January 2025 question paper for other subjects and for both shifts, the download is provided here:
| Parameter | Question Paper Link |
| Physics | JEE Main 2025 January 28 Shift 1, 2 Physics Question Paper with Answer Key Solutions |
| Mathematics | JEE Main 28 January 2025 Shift 1, 2 Mathematics Question Paper with Answer Key Solutions |
JEE Main 28 January 2025 Chemistry Question Paper PDFs by Coaching Institutions
Find the JEE Main 28 January 2025 Chemistry answer key and question paper PDFfor both Shift 1 and Shift 2, as released by Resonance, Aakash BYJU's, and Vedantu in the below table:
| Coaching Institute | Shifts | Chemistry Question Paper with Answer Key PDFs |
| Resonance | Shift 1 | Resonance JEE Main Chemistry Question Paper and Answer Key 2025 January 28 Shift 1 |
| Shift 2 | Resonance JEE Main Chemistry Question Paper and Answer Key 2025 January 28 Shift 2 | |
| Aakash | Shift 1 | Aakash JEE Main Chemistry Question Paper and Answer Key 2025 January 28 Shift 1 |
| Shift 2 | PDF download link to be updated soon | |
| Vedantu | Shift 1 | Vedantu JEE Main Chemistry Question Paper and Answer Key 2025 January 28 Shift 1 |
| Shift 2 | Vedantu JEE Main Chemistry Question Paper and Answer Key 2025 January 28 Shift 2 |
You may also find important |
| Parameter | Link |
| Safe Score | Expected Safe Score in JEE Mains January 2025 |
| Safe Percentile | Expected Safe Percentile in JEE Main 2025 Session 1 |
| Question Paper (All Days) | JEE Main Question Paper January 2025 Shift 1 and 2 |
| JEE Main Percentile to Marks | Expected Marks for JEE Main Percentile January 2025 |
| Cutoff Percentile | JEE Main Expected Cutoff Percentile January 2025 |
| Percentile Score | JEE Main Expected Percentile Score January 2025 |
| Cutoff Marks | Minimum Marks in JEE Mains to Qualify for JEE Advanced 2025 |
Subject-wise JEE Mains Marks vs Percentile 2025 |
| Name of the Subject | Link |
| Physics | JEE Main Physics Expected Marks vs Percentile 2025 |
| Chemistry | JEE Main Chemistry Expected Marks vs Percentile 2025 |
| Mathematics | JEE Main Mathematics Expected Marks vs Percentile 2025 |
| Event | Link for Expected/ Official Dates |
| Response Sheet | JEE Main Response Sheet January 2025 Expected Release Date |
| Answer Key | JEE Main Answer Key January 2025 Expected Release Date |
| Result | JEE Main Result Date January 2025 |
Keep visiting CollegeDekho for the latest Education News on entrance exams, board exams and admissions. You can also write to us at our email ID news@collegedekho.com.
