 Class 10 ICSE Chemistry Answer Key 2025 Live Updates: Unofficial key PDF download (Image Credit: Pexels)
Class 10 ICSE Chemistry Answer Key 2025 Live Updates: Unofficial key PDF download (Image Credit: Pexels)Class 10 ICSE Chemistry Answer Key 2025 : The ICSE Class 10 Chemistry Examination 2025 was conducted on March 21, 2025, and candidates are now awaiting the opportunity to assess their performance. To facilitate this, several reputable coaching institutes and subject experts are releasing unofficial answer keys, categorized by question paper sets, which are available for download in PDF format. The examination paper is traditionally divided into two sections: Section A, and Section B. After the examination concludes, the unofficial answer keys for these sections will be made available, providing candidates with an early opportunity to evaluate their performance. These are prepared by esteemed subject experts of CollegeDekho, enabling candidates to estimate their scores prior to the official result announcement.
Also Read | Class 10 ICSE Result Expected Release Date 2025
Class 10 ICSE Chemistry Answer Key 2025
Aspirants can check the unofficial answer key for the ICSE Class 10 Chemistry 2025 exam in the table below.
Section A Question 1
| Q. No. | Answers |
|---|---|
| Q1 (i) Which gas decolorises potassium permanganate (KMnO4) solution? | (a) Sulphur dioxide |
| Q1 (ii) Which formula represents a saturated hydrocarbon? | (b) C 5 H 12 |
| Q1 (iii) The metal whose oxide can be reduced by common reducing agents: | (a) Copper |
| Q1 (iv) An organic compound has a vapor density of 22. The molecular formula of the organic compound is (Atomic weight C=12. H=1) | (c) C 3 H 8 |
|
Q1 (v) In the reaction given below sulphuric acid acts as a/an:
S+2H 2 SO 4 →3SO 2 + 2H 2 O | (c) Oxidising agent |
|
Q1 (vi) Assertion (A): The tendency of losing electrons increases down the Group.
Reason (R): The most reactive metal is placed at the top of Group 1. | (d) (A) is false but (R) is true. |
| Q1 (vii) The ore that can be concentrated by using magnetic separation: | (b) Haematite |
Q1 (viii) The diagram given below shows the bonding in the covalent molecule AB
2
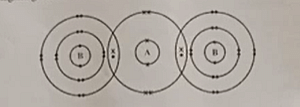 Which option represents the correct electronic configuration of atoms A and B before combining together to form the above molecule? |  |
| Q1 (ix) Which of the following options has all the compounds which are members of the same homologous series? | (a) CH 4 , C 2 H 6 , C 3 H 8 |
|
Q1 (x) Assertion (A): In the Contact Process SO
3
gas is not directly dissolved in water to obtain sulphuric acid.
Reason (R): Dense fog or misty droplets of sulphuric acid are formed which is difficult to condense. | (a) Both (A) and (R) are true, and (R) is the correct explanation of (A). |
|
Q1 (xi) Given below are four ions:
Cl
-
, Li
+
, Al
3+
, K
+
Identify the pair of ions which have the same electronic configuration. [Atomic number: Cl=17, Li=3, Al = 13, K=19] | (d) Cl - & K + |
| Q1 (xii) Which pair of reactants can be best used to produce lead (II) sulphate? | (c) Sodium sulphate + Lead nitrate |
|
Q1 (xiii) Aqueous copper (II) sulphate is electrolysed using copper electrodes.
Which statement about the electrolysis is not correct? (a) An oxidation reaction occurs at the positive electrode. (b) The current is carried through the electrolyte by ions. (c) The positive electrode loses mass. (d) The number of copper (II) ions in the electrolyte decreases. | (d) The number of copper (II) ions in the electrolyte decreases. |
| Q1 (xiv) X, Y & Z are three metallic atoms in successive order belonging to the same group such that atomic radii of 'X' is the smallest. Which of the three atoms is the best reducing agent? | (c) Z |
|
Q1 (xv) 40 cm³ of methane (CH
4
) is reacted with 60 cm³ of oxygen.
The equation for the reaction is given below:
CH
4
+ 2O
2
⟶ CO
2
+ 2H
2
O
All volumes are measured at room temperature.
hat is the total volume of the gases remaining at the end of the reaction? | (b) 40 cm³ |
Class 10 ICSE Chemistry Exam Analysis 2025 Link
Candidates can check out the ICSE 10th Chemistry Exam Analysis 2025 by clicking the link below.
Class 10 ICSE Subject-Wise Answer Key 2025 |
| Subject | Answer Key Link |
|---|---|
| English Language | Class 10 ICSE English Language Answer Key 2025 |
| English Literature | Class 10 ICSE English Literature Answer Key 2025 |
| Economics | Class 10 ICSE Economics Answer Key 2025 |
| Mathematics | Class 10 ICSE Mathematics Answer Key 2025 |
| Hindi | Class 10 ICSE Hindi Answer Key 2025 |
| History & Civics | Class 10 ICSE History & Civics Answer Key 2025 |
| Geography | Class 10 ICSE Geography Answer Key 2025 |
| Physics | Class 10 ICSE Physics Answer Key 2025 |
| Chemistry | Class 10 ICSE Chemistry Answer Key 2025 |
| Biology | Class 10 ICSE Biology Answer Key 2025 |
Students should stay tuned to live updates for the latest information on expert analysis, difficulty level, and real-time updates.
Class 10 ICSE Chemistry Answer Key 2025 Live Updates
08 00 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Most Time Consuming Section
Time-Consuming Section (If any)
None
07 40 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Lengthiest Question
Lengthiest Question (If any)
No, Mostly questions were doable
07 20 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Section-wise Difficulty Level
Difficulty Level of Section A
Easy to Moderate
Difficulty Level of Section B
Easy to Moderate
07 00 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Overall Good Attempts
The expected good attempts for the Chemistry paper are 65+.
06 40 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Overall Exam Level of Difficulty
The Chemistry paper was of Easy to Moderate level as per initial feedback of students.
06 20 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (xv)
Q1 (xv) 40 cm³ of methane (CH4) is reacted with 60 cm³ of oxygen.
The equation for the reaction is given below:CH4 + 2O2 ⟶ CO2 + 2H2O
All volumes are measured at room temperature.
hat is the total volume of the gases remaining at the end of the reaction?(b) 40 cm³ 06 00 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (xiv)
Q1 (xiv) X, Y & Z are three metallic atoms in successive order belonging to the same group such that atomic radii of 'X' is the smallest. Which of the three atoms is the best reducing agent? (c) Z 05 40 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (xiii)
Q1 (xiii) Aqueous copper (II) sulphate is electrolysed using copper electrodes.
Which statement about the electrolysis is not correct?
(a) An oxidation reaction occurs at the positive electrode.
(b) The current is carried through the electrolyte by ions.
(c) The positive electrode loses mass.
(d) The number of copper (II) ions in the electrolyte decreases.(d) The number of copper (II) ions in the electrolyte decreases. 05 20 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (xii)
Q1 (xii) Which pair of reactants can be best used to produce lead (II) sulphate? (c) Sodium sulphate + Lead nitrate 05 00 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (xi)
Q1 (xi) Given below are four ions: Cl- , Li+, Al3+ , K+
Identify the pair of ions which have the same electronic configuration. [Atomic number: Cl=17, Li=3, Al = 13, K=19](d) Cl- & K+ 04 40 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (x)
Q1 (x) Assertion (A): In the Contact Process SO3 gas is not directly dissolved in water to obtain sulphuric acid.
Reason (R): Dense fog or misty droplets of sulphuric acid are formed which is difficult to condense.(a) Both (A) and (R) are true, and (R) is the correct explanation of (A). 04 20 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (ix)
Q1 (ix) Which of the following options has all the compounds which are members of the same homologous series? (a) CH4, C2H6, C3H8 04 00 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (viii)
Q1 (viii) The diagram given below shows the bonding in the covalent molecule AB2 
Which option represents the correct electronic configuration of atoms A and B before combining together to form the above molecule?
03 40 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (vii)
Q1 (vii) The ore that can be concentrated by using magnetic separation: (b) Haematite 03 20 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (vi)
Q1 (vi) Assertion (A): The tendency of losing electrons increases down the Group.
Reason (R): The most reactive metal is placed at the top of Group 1.(d) (A) is false but (R) is true. 03 00 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (v)
Q1 (v) In the reaction given below sulphuric acid acts as a/an:
S+2H2SO4→3SO2 + 2H2O(c) Oxidising agent 02 45 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (iv)
Q1 (iv) An organic compound has a vapor density of 22. The molecular formula of the organic compound is (Atomic weight C=12. H=1) (c) C3H8 02 30 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (iii)
Q1 (iii) The metal whose oxide can be reduced by common reducing agents: (a) Copper 02 15 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (ii)
Q1 (ii) Which formula represents a saturated hydrocarbon? (b) C5H12 01 55 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Q1 (i)
Q1 (i) Which gas decolorises potassium permanganate (KMnO4) solution? (a) Sulphur dioxide 01 30 PM IST - 21 Mar'25
10th ICSE Chemistry Answer Key 2025: Exam Analysis Soon
Based on the initial feedback shared by the candidates, 10th ICSE Chemistry exam analysis 2025 will be shared on the page shortly.
01 15 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Answer Key Soon
Our subject experts will share the unofficial 10th ICSE Chemistry Answer Key 2025 soon.
01 00 PM IST - 21 Mar'25
10th ICSE Chemistry Answer Key 2025: Exam Over
The final bell has rung. ICSE Class 10 Chemistry 2025 examination is over. Candidates are submitting their answer sheets.
12 40 PM IST - 21 Mar'25
ICSE 10th Chemistry Answer Key 2025: Previous Year Question 2
Ques: Hydrogen chloride gas is prepared in the laboratory by the action of concentrated sulphuric acid on sodium chloride.
Options:
(a) Give balanced chemical equation for the above reaction.
(b) State the method of collection of the gas formed above.
(c) What is property of sulphuric acid that makes it a suitable reagent for the reaction.
12 20 PM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Previous Year Question 1
Ques: Copper, Zinc, and Tin are the metals alloyed to form:
Options:
(a) Duralumin
(b) Brass
(c) Bronze
(d) Solder
12 00 PM IST - 21 Mar'25
ICSE Class 10 Chemistry Answer Key 2025: Final One Hour Left
The last 1 hour is left for the paper to finish. Candidates must speed up, tie up their papers, and continue writing.
11 40 AM IST - 21 Mar'25
ICSE 10th Chemistry Answer Key 2025: Marking Scheme
There is no negative marking on the paper. Candidates will get marks as suggested for each Section.
11 20 AM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Question Paper Pattern
The question paper comprises two sections - Section A and Section B. Section A comprises Question 1 which includes 15 sub-questions of 1 mark each, and Question 2 which includes 5 sub-questions of 5 mark each. Section B comprises Questions 3, 4, 5, 6, 7, and 8 which includes 4 sub-questions each, each carrying 2 to 3 marks.
11 00 AM IST - 21 Mar'25
10th ICSE Chemistry Answer Key 2025: Exam Starts
As per the schedule, 10th ICSE Chemistry 2025 examination has now begun. Candidates must start answering their papers.
10 40 AM IST - 21 Mar'25
ICSE Class 10 Chemistry Answer Key 2025: Question Paper Distributed
The question paper is being distributed to the students. Post-distribution, candidates will have 15 minutes to go through the paper thoroughly.
10 20 AM IST - 21 Mar'25
ICSE 10th Chemistry Answer Key 2025: Revision Questions 8
Name the main metal used in making of alloys given below:
(a) Duralumin
(b) Stainless steel
10 00 AM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: One Hour to Go
Only one hour is left for the exam to start. Candidates must take their seats, and get ready to answer the paper.
09 45 AM IST - 21 Mar'25
ICSE Class 10 Chemistry Answer Key 2025: Revision Questions 7
Complete the following sentence:
(a) The salt that can be prepared by Direct Combination is ______.
(b) The metallic oxide which can be reduced by using common reducing agents is _________.
(c) The metal nitrate which on thermal decomposition forms a black residue is _______.
(d) During the electrolysis of copper sulphate solution, if ______ is used as an electrode, the colour of the electrolyte does not fade.
(e) The process of heating the concentrated ore in a limited supply or absence of air is _______.
09 30 AM IST - 21 Mar'25
ICSE 10th Chemistry Answer Key 2025: Revision Questions 6
Ques: Ammonia gas is collected by downward displacement of air since ammonia is:
Options:
(a) very slightly soluble in water.
(b) heavier than air.
(c) lighter than air.
(d) insoluble in water.
09 15 AM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Revision Questions 5
Give balanced equation for the following reactions:
(a) Copper reacts with concentrated Nitric acid.
(b) Aluminium nitride is treated with warm water
09 00 AM IST - 21 Mar'25
ICSE Class 10 Chemistry Answer Key 2025: Revision Questions 4
Give reasons for the following:
(a) Inert gases do not form ions.
(b) Covalent compounds have a low melting and boiling point.
08 45 AM IST - 21 Mar'25
10th ICSE Chemistry Answer Key 2025: Revision Questions 3
Ques: The property exhibited by concentrated sulphuric acid when it is used to prepare hydrogen chloride gas from potassium chloride:
Options:
(a) Dehydrating property
(b) Drying property
(c) Oxidizing property
(d) Non-volatile acid property
08 30 AM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: Revision Questions 2
Ques: The compound that is not one of aluminium:
Options:
(a) Cryolite
(b) Corundum
(c) Fluorspar
(d) Bauxite
08 15 AM IST - 21 Mar'25
ICSE Class 10 Chemistry Answer Key 2025: Revision Questions 1
Give reason for the following:
(a) lonisation potential decreases down a group.
(b) lonic compounds do not conduct electricity in solid state.
08 00 AM IST - 21 Mar'25
Class 10 ICSE Chemistry Answer Key 2025: When will the exam start?
As per the schedule, Class 10 ICSE Chemistry exam 2025 will be held today, March 21, 2025, from 11 AM to 13 PM.
Are you feeling lost and unsure about what career path to take after completing 12th standard?
Say goodbye to confusion and hello to a bright future!

Was this article helpful?





 Follow us
Follow us













