Some Basic Concepts of Chemistry
Symbolically, Chemistry may be defined as the science of Rasas and colours. It colours our life with the discovery of different hidden hues of nature and all rasas necessary for making our life happier. The food we eat, the medicines we use for the prevention and cure of diseases, the natural and synthetic fibres used for making fabrics, the various types of polymers used in all walks of life, the cosmetics we use, the essences, the dye stuffs, all kinds of fuels used from kitchen stoves to rockets, all metals, non-metals and many other things.
So all in all, Chemistry deals with the study of materials, their preparations, their properties, their accurate super-microscopic structures, their mutual interactions and their use in all possible walks of life.
Importance and Scope of Chemistry
The scope of chemistry is practically unlimited. It finds applications in all walks of life. Since its origin, chemistry has served mankind. Perhaps, no other branch of science has done so much to make a man’s life happier as chemistry did. Whatever we see around us and whatever we use in our day-to-day life is related to chemistry in some way or the other. Chemistry has penetrated in our life to such an extent that we cannot think of a smooth running life without it.
Both at the level of necessity and at the level of luxuries, chemistry has proved itself to be the best friend of mankind. The contribution of chemistry to agriculture is very significant. It developed new varieties of seeds. The chemical fertilizers, insecticides, pesticides, etc., and improved quality of seeds have brought a green revolution all over the world. The promises of chemistry in this field are so encouraging that it will completely eradicate starvation from the world in near future. Chemistry has provided synthetic fibers to make cheaper and better looking clothes. Chemistry has been successful in providing a wide range of medicines to fight against all kinds of diseases. Many life saving drugs such as cisplatin, taxol, Azidothymidine (AZT), etc., are playing a vital role in combating serious diseases like cancer, AIDS, etc. Polymers are being used for a variety of purposes in all walks of life. The electronics and electrical industries lean heavily upon chemistry for the basic material used therein. It has provided various types of fuels for various purposes. Chemistry is also conscious about the increasing pollution on this planet. It is trying hard to check pollution and to make the environment healthier.
Quick Links for Physics Preparation:
Nature of Matter
The properties of matter can be classified as (i) Physical Properties and (ii) Chemical Properties.
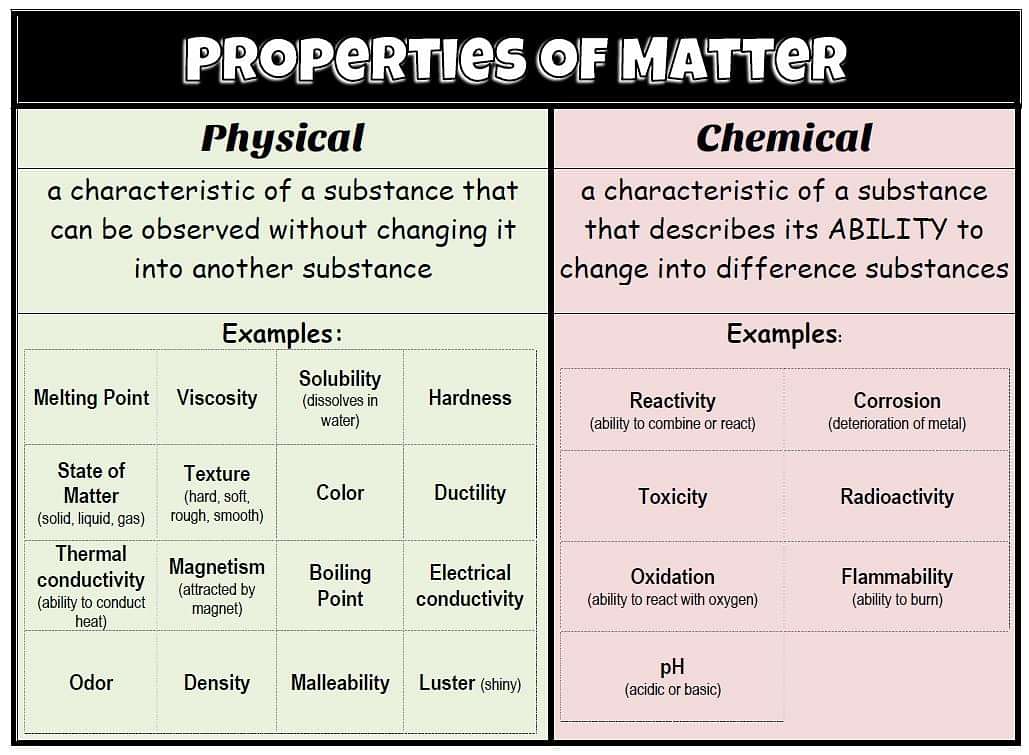
Physical Properties are those properties which can be measured without altering the identity of the substance. Colour, odour, density, melting point, boiling point, specific heat, etc., are some common examples of physical properties.
Chemical Properties are those which require a chemical change and involve a change in the chemical composition of the substance. The examples of chemical properties are the characteristic reactions of the substances, eg., Combustion, Reaction with acids, reaction with alkalis, etc.
Laws of Chemical Combination
Chemistry deals mainly with the reactions. In Fact reactions are the essence of chemistry. In the eighteenth century, chemists found that all chemical reactions always take place in accordance with certain laws. These laws are referred to as laws of chemical combination. A brief discussion of these laws is as follows:
-
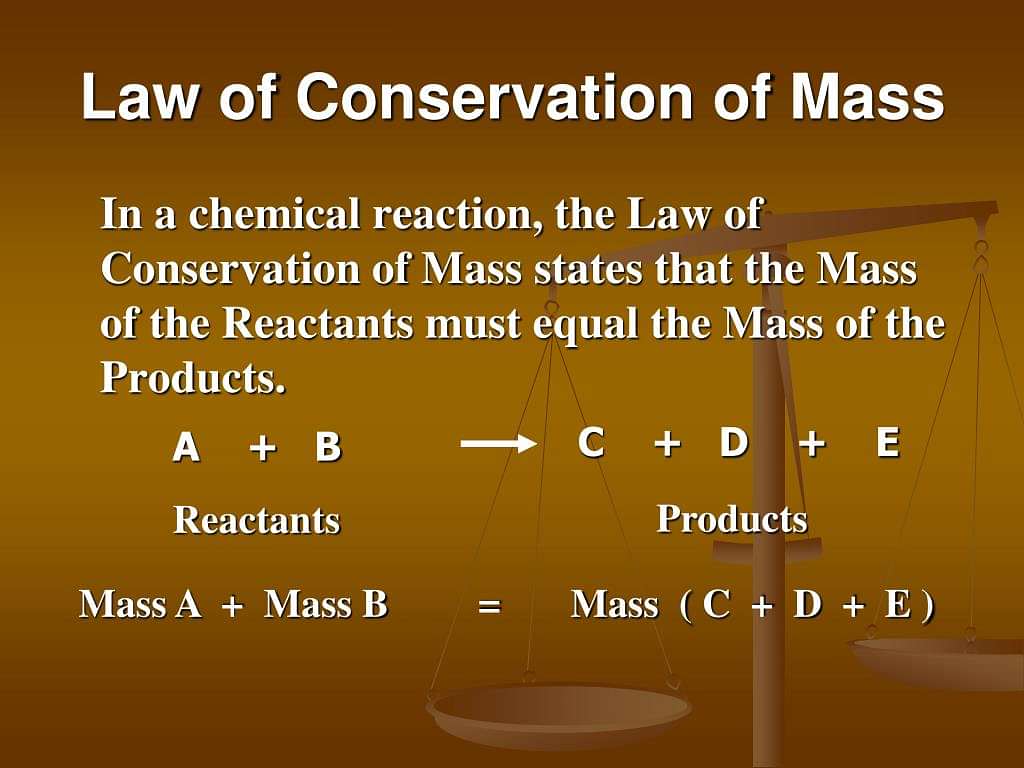
Law of Conservation of Mass: During any physical or chemical change, the total mass of the product obtained is always equal to the mass of the reactants taken at the beginning of the change. This signifies that during any physical or chemical change, the matter is neither created nor destroyed. However, it may change from one state to another.
-
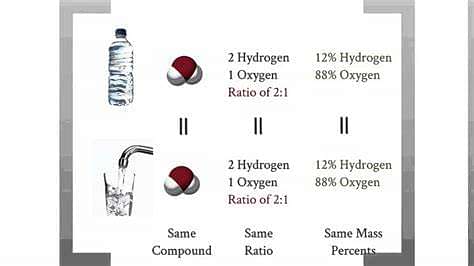
Law of Constant Composition: A chemical compound, no matter from which source it is obtained, always contains the same elements combined together in the same definite proportion by mass.
-
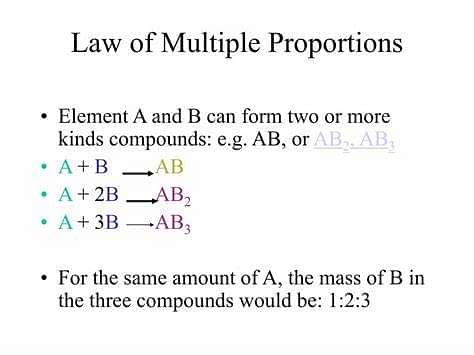
Law of Multiple Proportion: When two elements combine to form two or more compounds, then the different masses of one element, which combine with a fixed mass of the other element, bear a simple whole number ratio with one another.
-
Law of Reciprocal Proportion: When two elements combine separately with a definite mass of a third element, then the ratio of their masses in which they do so is either the same or some whole number multiple of the ratio in which they combine with each other.
- Gay Lussac’s law of combining volumes: When two or more gases react with one another, they do so in volumes, which bear a simple whole number ratio with one another and to the volume of products (if they are also gases) provided all the volumes are measured under identical conditions of temperature and pressure.
Dalton’s Atomic Theory: Concept of elements, atoms and molecules
Chemical reactions always obey certain laws known as Laws of chemical combination. These laws suggest that the matter is composed of discrete particles which do not break up during a chemical change. John Dalton, an English Chemist, pondered over these laws and suggested that the discrete particles which build up matter are atoms. On the basis of the laws of the chemical combination and the work of ancient Greek philosophers, he proposed a very important theory known as Dalton’s Atomic Theory in the year 1804. The basic postulates of this theory area as follows:
- Matter is made of extremely small discrete particles called atoms.
- Atoms of a particular element are identical in all respects, i.e, they possess the same size, same shape, same mass and similar chemical properties.
- Atoms of one element differ from atoms of other elements in all respects, i.e, the atoms of two elements differ in size, shape, mass and chemical properties.
- Atoms are indivisible, i.e, they cannot be further subdivided.
- Atoms are indestructible, i.e, they can neither be created nor be destroyed.
- Atom is the unit of the chemical reaction, i.e, it is the smallest particle that takes part in a chemical reaction.
- Atoms of different elements combine with each other in a fixed, simple, whole number ratio to form molecules.
Atomic and Molecular Masses
The atom of an element is an extremely small particle and is indivisible. This poses a big problem in the determination of its absolute mass. The atom is so small that it cannot be weighed even with the help of the most sensitive balance known till today. Hence, determination of mass of an atom by some weighing technique is not feasible. Several indirect and advanced techniques have been used for the determination of absolute masses of atoms. An idea of how small the atom is, can be had by the fact that the absolute atomic mass of an atom of hydrogen is 1.67 x 10-24 g. The absolute atomic masses of other atoms are also of the same order. Since the absolute atomic masses of all atoms are extremely small numbers, it is very inconvenient to use them in routine calculations. Hence, it was decided to use relative masses instead of absolute masses in routine calculations.
The relative atomic mass of an atom is its absolute mass compared to the absolute mass of an atom of a standard substance.
Thus,
Relative atomic mass = Absolute mass of an atom of the given substance
Absolute mass of an atom of the standard substance
In 1961, C12 (the most stable isotope of carbon) was adopted by the International Union of Chemists to calculate the relative atomic masses of elements. And on the basis of this,
The Relative Atomic Mass of an element is the relative mass of an atom of that element as compared to 1/12 th the mass of an atom of carbon (C12).
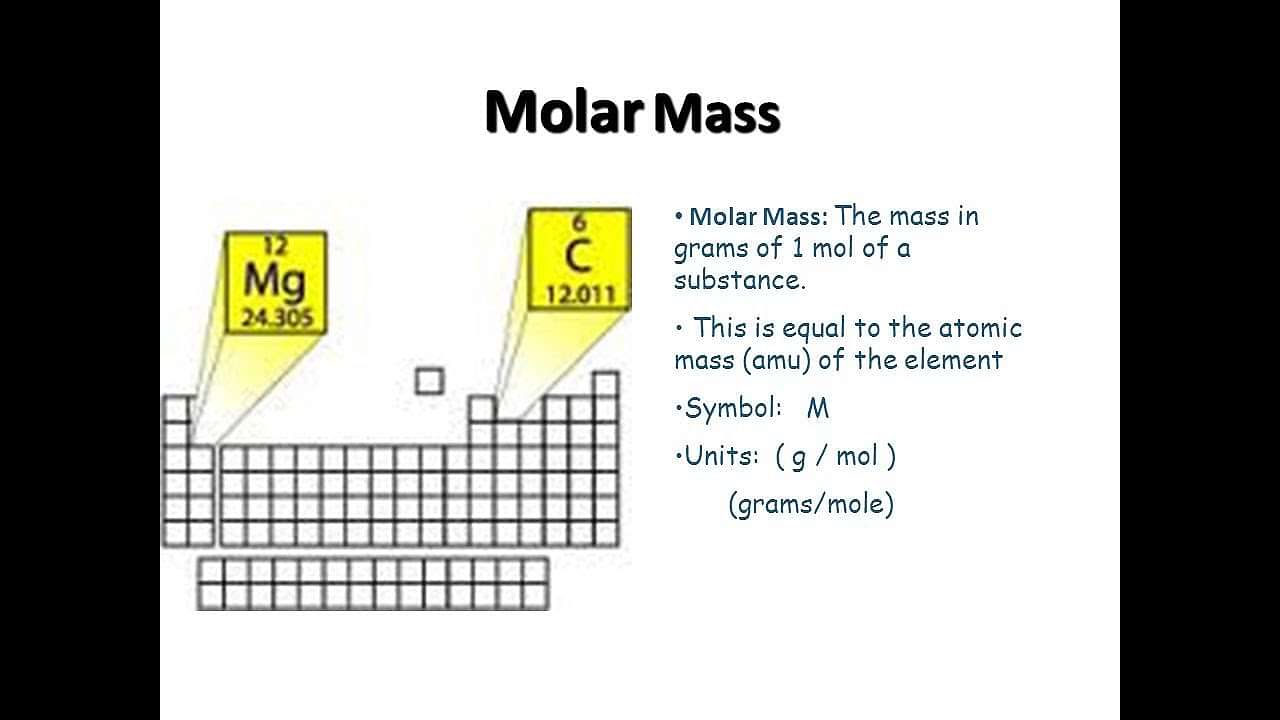
Mole Concept and Molar Mass
Even after defining the atomic mass of an element in terms of an arbitrarily assumed mass of a standard substance, the problem of knowing the absolute or actual mass of an atom still remains unsolved. This problem was solved by introducing the Mole Concept.
As per the mole concept, the number of atoms present in one gram of atom of any element is the same as the number of molecules present in one gram molecule of any substance and is equal to the number of atoms present in 12 g of C12. On calculating, this number was found to be significant and its value was found to be 6.022 X 1023. This value is also known as the Avogadro Number.
There, A mole is the amount of any substance which contains as many as elementary entities (atoms, molecules and ions) as there are atoms in exactly 0.012 Kg (i.e 12 g) of carbon C12.
Molar Mass: The mass of one mole of a particular type of elementary entity (atoms, molecules or ions) expressed in grams is called the molar mass of that type of entity. It is actually the mass (in grams) of 6.022 X 1023 elementary entities.
Percentage Composition
Volumetric analysis is a quantitative analysis and involves the determination of the strength of an unknown solution with the help of a solution of known strength. The procedure used is called Titration. Titration involves the addition of a solution of known strength to a definite volume of a solution of unknown strength until the reaction between the two is just complete.
Composition or Strength of a solution (Terms used in Volumetric Calculation)
The amount of solute dissolved in a particular amount of solvent is known as composition or strength or concentration of a solution. It can be expressed in several units. Some of the important ones are given below:
- Mass Percentage or Percentage by Mass (w/w)
Mass Percentage is defined as the mass of the solute in grams dissolved per 100 g of the solution. It is also referred to as weight percentage (w/w).
2. Volume Percentage or Percentage by Volume (v/v)
Volume Percentage is defined as the volume of a solute (in a particular unit) present in the 100 units of the volume of the solution.
3. Grams Per Litre (g/L)
The concentration of a solution in grams per litre refers to the amount of solute in grams present in one litre of solution.
4. Molarity (M)
This is an important unit widely used to express the composition of a solution. It is represented by M and is defined as follows:
The number of moles of solute dissolved per litre of the solution at a particular temperature is called the molarity of the solution at that temperature.
5. Formality (F)
This unit is used for ionic compounds which exist in the form of aggregates formed by the ions in solution. Actually, ionic compounds do not possess discrete molecules and the term molecular mass has no meaning for them. Hence, a new unit of concentration called formality has been introduced for ionic compounds. It is represented by F and is defined as follows:
The number of gram formula mass of an ionic solute dissolved per litre of solution at a particular temperature is called Formality of the solution.
6. Molality (m)
Molality of a solution is defined as the number of moles of solute dissolved per 1000g (1 Kg) of the solvent. It is represented by m. Molality actually represents the concentration of a solution in mol per kg.
7. Normality (N)
It is defined as the number of gram equivalents of the solute dissolved per litre of a solution at a particular temperature is called the normality of the solution. It is represented by the symbol N. It actually expresses the concentration of a solution in gram equivalents per litre.
8. Mole Fraction
The mole fraction of a particular component in a solution is the ratio of the number of moles of that component to the total number of moles of all the components present in the solution. Mole fraction is independent of temperature.
Empirical and Molecular formula
The chemical Formula of compounds may be of following two types:
(i) Empirical formula: This formula gives the simple whole number ratio of different types of atoms present in one molecule of the compound. For example, the empirical formula of benzene is CH. It simply represents that carbon and hydrogen atoms are present in the ratio 1:1 in benzene. Similarly, the empirical formula of glucose is CH2O which tells that carbon, hydrogen and oxygen atoms are present in the ratio 1:2:1 in a molecule of glucose.
(ii) Molecular Formula: This formula represents the actual number of different types of atoms present in one molecule of the given compound. For example, the molecular formula of benzene is C6H6. This indicates that one molecule of benzene is composed of 6 atoms of carbon and 6 atoms of hydrogen. Similarly, the molecular formula of glucose is C6H12O6 which implies that one molecule consists of 6 atoms of carbon, 12 atoms of hydrogen and 6 atoms of oxygen.
Chemical Reactions
In chemistry, we mainly deal with the mutual interaction of substances. The mutual interaction of one substance with the other is named as chemical reaction. A chemical reaction may involve several reactants and products. Since we study chemical reactions very frequently, it is necessary to represent a chemical reaction in a concise form. The brief representation of a chemical reaction is made in the form of a chemical equation which involves the chemical formulae of all reactants and products in stoichiometric ratio involved in the reaction. Reactants are those substances which react among themselves to bring about the chemical reaction while products are those substances which are produced on account of the chemical reaction. Stoichiometric ratio is the simplest whole number ratio of moles of reactants and products involved in the reaction. A chemical equation may be defined as follows:
A chemical equation is a brief representation of a chemical reaction in terms of a chemical reaction in terms of chemical formulae of reactants and products in stoichiometric ratio.
Stoichiometry and Calculations based on it
The brief representation of a chemical reaction is made in the form of a chemical equation which involves the chemical formulae of all reactants and products in stoichiometric ratio involved in the reaction. Stoichiometric ratio is the simplest whole number ratio of moles of reactants and products involved in the reaction.
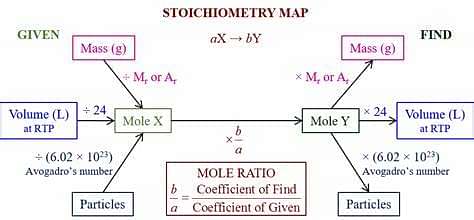
For example, let us consider a simple reaction in which zinc reacts with dilute hydrochloric acid to form zinc chloride and hydrogen gas. This reaction can be represented in the form of a chemical equation as follows:
This equation gives almost complete information about the reaction under consideration. It says that one mole of zinc reacts with the two moles of dilute hydrochloric acid to form one mole of zinc chloride and one mole of gaseous hydrogen. The whole numbers representing the moles of reactants and products involved in the reaction are stoichiometric coefficients. In the above equation, the stoichiometric coefficients are 1 (for Zn) and 2 (for Zncl2).
Calculations based on Chemical Equations (Stoichiometry)
The stoichiometric calculations are found to be very useful in exploring the economic viability of a chemical process. The yield of a particular process, the amount of reactants needed to obtain a particular product, the volumes of gases evolved etc. can easily be predicted with the help of these calculations.
Following examples illustrate how the stoichiometric calculations should be carried out in a systematic way.
Ques 1. How much zinc should be treated with excess dilute hydrochloric acid to obtain 2.24 litres of hydrogen at S.T.P.
Solution. The balanced chemical equation representing the combination of zinc with hydrochloric acid is
Zn + 2 HCl → ZnCl2 + H2
This equation signifies that 1 mole i.e. 65.38 g of zinc reacts with hydrochloric acid to produce 1 mole i.e 22.4 L of hydrogen at S.T.P
Since, 22.4 L of hydrogen at S.T.P are given by zinc = 65.38 g
Therefore, 2.24 L of hydrogen at S.T.P will be given by zinc
65.38/ 22.4 x 2.24 = 6.54 g
Hence, 2.24 L of hydrogen at S.T.P will be produced by the action of 6.54 g of zinc with the excess of HCl.
















Similar Articles
IGNOU B.Ed Admission 2025
Karnataka University B.Ed Syllabus 2024-25 Semester-Wise
HTET 2024: Exam Dates (Out), Application Form, Syllabus, Pattern, Result, Selection Process
Karnataka B.Ed Admission 2024: Registration, Application Form, Counselling
Karnataka B.Ed Selection List 2024: Date, Seat Allotment Result Link, PDF Download
Assam TET 2025: Dates, Application Form, Syllabus, Result, Selection Process