
Kinetic Theory
The properties of gases can be explained on the basis of the kinetic model of gas. According to this model, every gas is made up of minute particles called ‘molecules’. All the molecules of a pure gas are identical. The gas molecules are at a large distance apart from each other. We know that 1 cm3water is converted into 1700 cm 3 of steam on boiling. This means that in 1700 cm 3 steam the volume of molecules is only 1 cm 3 . Thus, most of the space in a gas is empty. In this space, the molecules of the gas are moving rapidly in straight lines in all possible directions. During motion, the molecules constantly collide with one another and with the walls of the containing vessel, and their speeds and directions of motion are changing continuously.
The large-scale action of the gas can be easily studied and measured, however, to study the action of the gas molecules a theoretical model needs to be used. This theoretical model is known as the kinetic theory of gases. In this article let us discuss the kinetic theory of gases and the assumptions considered for the theory.
Equation of state of a perfect gas
The study of gases shows that the volume, pressure and temperature of a given mass of a gas are inter-related to one another. If we fix the values of any two of them, then the value of the third cannot be changed. However, there is a relation between the pressure, volume and temperature of a given mass of a gas. This relation is called the ‘perfect gas equation’.
Suppose, initially the pressure, volume and absolute temperature of a given mass of a gas are P 1 , V 1 and T 1 respectively and they finally change to P 2 , V 2 and T 2 respectively. We can divide this change into two parts:
- Suppose the temperature of the gas is kept constant at T 1 and the pressure is changed from P 1 to P 2 . If in this process the volume changes from V 1 to V’ , then by Boyle’s law, we have P 2 V' = P 1 V 1
V' = P 1 V 1 / P 2
- Now, suppose the pressure of the gas is kept constant at P 2 and its absolute temperature is changed from T 1 to T 2 . If in this process, the volume of the gas changes from V' to V 2 , then by Charles’s law, we have
V'T 1 = V 2 T 2
Or V' = T 1 V 2 / T 2
From both these equations, we have
P 1 V 1 / T 1 = P 2 V 2 / T 2
Hence, for a gas, the value of PVT = r
Or PV= rT
This is the perfect gas equation. The value of the gas-constant r depends upon the nature and the mass of the gas. Its value is different for different masses of the same gas or for different gases of the same mass. It is also called ‘specific gas constant’. However, if we take 1 gram molecule (1 mole) of a gas, the value of gas constant will be the same for all gases. Then it is called ‘universal gas constant’ and is represented by R. Hence for 1 mole of gas, the gas equation takes the following form:
PV= RT
Also Read:
Kinetic theory of gases - Assumptions
In order to calculate the pressure of the gas on the basis of the kinetic theory, the following assumptions are made which are true only for an ideal gas:
- All the molecules of a gas are identical, rigid and very small in size. The volume of the molecules themselves is negligible compared to the volume occupied by the gas.
- Normally no force acts between the molecules. Hence, they move in straight lines at constant speeds. But, when two molecules happen to come very close to each other, a repulsive force begins to act between them due to which the speeds and the directions of motion of the molecules change and new straight line motion starts. This event is called a collision between two molecules. Thus, between two collisions the molecules travel in straight lines with constant s[eeds. The average distance travelled by a molecule between two successive collisions is called the mean free path of the molecule.
- The collision between two molecules is perfectly elastic, that is, the kinetic energy remains conserved in the collision.
- The duration of a collision is negligible compared with the time spent by a molecule between collisions.
- Because the molecules inside the vessel keep on moving continuously in all possible directions, the distribution of molecules in the whole vessel remains uniform.
- Because the mass of a molecule is negligibly small and the speed is very large, there is no effect of gravity on the motion of the molecules. If this effect were there, the density of the gas would have been greater at the bottom of the vessel.
Concept of pressure
When a gas is enclosed in a vessel, it exerts pressure on the walls of the vessel. According to the kinetic theory of gases, this pressure is developed due to the collisions of the moving molecule on the walls of the vessel. Whenever a molecule collides with the wall, it returns with a changed momentum, and an equal momentum is transferred to the wall (conservation of momentum). According to Newton’s law of motion, the rate of change of momentum of the wall is equal to the force exerted on the wall. Since the gas contains a very large number of molecules which are colliding with the walls of the vessel, they exert a steady force on the walls. This force is given by the average rate of change of momentum. The force measured per unit area of the wall is called the ‘Pressure’ of the gas.
P = 13mnVv2
This is the expression for the pressure of the gas, v2 is called the mean square velocity of the molecules. Since mn is the mass of the whole gas and V is the volume, so mnV is the density ⍴ of the gas. Therefore, the above expression can be written as
P = 13⍴ v2
Kinetic Interpretation of Temperature
The temperature of a body is the measurement of its hotness or coldness. If, on touching, body A appears hotter than body B, then A is at a higher temperature than B. If these bodies are placed in mutual contact, heat flows from body A to body B until the temperature of both becomes equal. After this, the flow of heat from A to B stops and ‘thermal equilibrium’ is established between A and B. Thus, bodies between which there is no exchange of heat are said to be in thermal equilibrium, and they are at the same temperature.
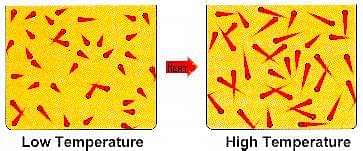
In kinetic theory of gases, the interpretations of temperature and thermal equilibrium are based on the velocity and energy of the gas molecules.
Suppose 1 mole of a gas is at an absolute temperature T and has a volume V. The number of molecules in 1 mole if a gas is N ( = 6.02 X 1023) which is called the ‘Avogadro’s Number’. Let the mass of a molecule of the gas be m and the mean square velocity of the molecules be v2. The, according to the kinetic theory, the pressure of 1 mole of the gas is given by
But mN = mass of one molecule X number of molecules in 1 mole of the gas = mass of 1 mole of the gas molecular weight M.
∴ PV = ⅓ M v2
The ideal-gas equation for 1 mole is PV = RT, where R is a gas-constant
∴ RT = ⅓ M v2
or v2 = 3RTM
Taking square root: √v2 = 3RTM
Therefore, v rms ∝ T
Thus, the root mean square velocity of the molecules of a gas is directly proportional to the square - root of the absolute temperature of the gas. This means that faster the motion of the molecules of a gas, higher will be the temperature of the gas. This means that faster the motion of the molecules of a gas, higher will be the temperature of the gas. This is the kinetic interpretation of temperature.
It must be remembered that the temperature of a gas is related to the translatory motion of the molecules of the gas with respect to the center of mass of the gas, and not with the motion of the center of mass of the gas itself. If we place a vessel filled with gas in a fast moving train, the temperature of the gas will not rise.
It is also evident from the above relation that if the absolute temperature of the gas becomes zero (T=0), then the motion of its molecules will cease ( v rms = 0). Hence, according to the kinetic theory, absolute zero is that temperature at which the motion of all the molecules of the gas stops. It is also clear that absolute temperature can never be negative, because v rms can never be negative. Thus, no temperature is possible below absolute zero.
Also Read:
- Physics Class 12 Laws of Motion
- Physics Class 12 Mechanical Properties of Fluid
- Physics Class 12 Mechanical Properties of Solid
Rms speed of gas molecules
The velocity of individual molecules of a gas vary over a wide range. Let v1, v2, v3 ........vn be the velocities of n molecules of a gas. Then, the root mean square velocity of the molecules is defined as the square root of the mean of the squares of the velocities of all the gas molecules, and is denoted by v rms. Thus ,
We know that the pressure of a gas is given by
P = ⅓ ρ v2
v rms = √v2 = √3P/ρ
Degrees of freedom
The degrees of freedom of a particle indicate the number of independent motions which the particle can undergo, or the number of independent methods of exchanging energy.
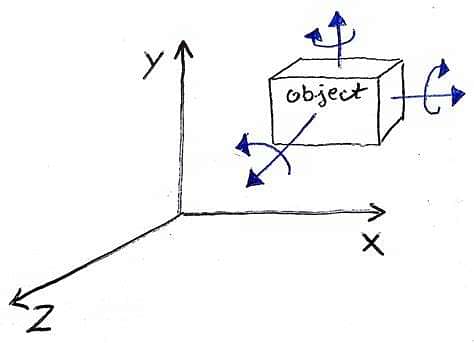
The molecule of a monatomic gas (such as He, A, etc.) consists of a single atom. Its transitional motion can take place in any direction in space. Thus, it can be resolved along three coordinate axes and can have three independent motions. Hence, it has three degrees of freedom, all transnational. A monatomic molecule can rotate also. But it has such a small moment of inertia that its kinetic energy of rotation is insignificant. Therefore, it possesses only kinetic energy of translation.
The molecule of a diatomic gas (such as H2, O2, etc.) is made up of two atoms joined rigidly to one another through a bond. This can not only move bodily, but also rotate about any of the three coordinate axes. However, its moment of inertia about the axis joining the two atoms is negligible. Hence, it can have only two rotational motions. Thus, a diatomic molecule has five degrees of freedom, three with respect to transnational and two with respect to rotation.
The diatomic molecule can vibrate also along the line joining the two atoms. But, usually, vibrational motion does not occur at ordinary temperatures.
A polyatomic molecule (such as H2O) can rotate about any of the three coordinate axes. Hence, it has six degrees of freedom, three transnational and three rotational. If the atoms of the molecule vibrate with respect to each other, it would possess vibrational degrees of freedom also).
Law of Equi-Partition of Energy and Application to Specific Heat Capacities of Gases

According to this law, in a classical system of particles which is in equilibrium at absolute temperature, T, the average internal (kinetic) energy per article associated with each degree of freedom is ½ kT, where k is the boltzmann’s constant. If the particle has f degrees of freedom, its average kinetic energy would be ½ fk T.
Concept of Mean Free Path
The molecules of a gas do not trace a straight line motion. The reason for this is the fact that the molecules of the gas collide with each other and change speed and direction simultaneously. So just before colliding with another molecule, a particular molecule travels a path.
Then between the first and the second collision also, that particular molecule travels a certain distance. The average of all these path lengths between two successive collisions is defined as the mean free path. In this article, we will derive an expression for the mean free path.
Avogadro's number
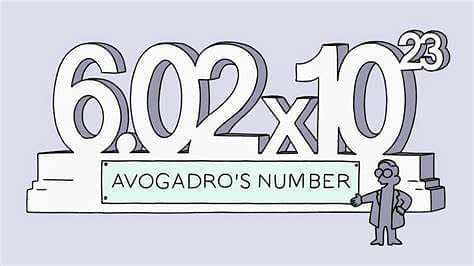
The number of elementary entities that make up one mole of any substance is termed Avogadro's number. Furthermore, it is the number of atoms that are found to be in exactly 12 grams of carbon-12. Moreover, this value is experimentally determined and it turns out to be approximately 6.0221 x 1023 particles per mole.
















Similar Articles
DHE/SAMS Odisha +3 Admissions 2025: Registration, Eligibility Criteria, Selection Process, Seat Allotment, Merit List
B.Ed Admission 2025: Dates, Application Form, Eligibility, Selection Process, Fees, Top Colleges
List of Documents Required to Fill UGC NET 2024 Application Form: Image Upload, Specifications
REET Syllabus 2025 for Level 1 and 2: Download Subject-wise PDFs, Exam Pattern
Karnataka B.Ed Selection List 2024: Date, Seat Allotment Result Link, PDF Download
TS TET 2024: Exam Dates (Out), Registration (Closed), Admit Card, Results