Units and Measurements hold a great weightage in Physics for all the science stream students. Check out the notes on Units and Measurements, Types of Units, System of Units, and Dimensional Analysis here.
- Need For Units and Measurements
- Types of Units of Measurement
- Units and Measurements: Systems of Units
- Units and Measurements - Fundamental Units
- Units and Measurements - Derived Units
- Significant Figures in Units and Measurements
- Dimensions Of Physical Quantities
- Dimensional Analysis
- Units and Dimensions of Derived Quantities
- Units and Measurements - Important Conversions Formulas
- Units and Measurements - Important Physical Constants
- Units and Measurements - Some Important Questions With Solutions

Units and Measurements: There are 7 base units that are available in the International System of units for all of the physical quantities. Units and measurements are one of the most important aspects of the class 12th physics subject because it carries most of the weightage in the examination. There are various subdivisions that are included in the chapter such as the dimensional analysis and the derived units other than the base units. The quantities are there for the students to describe the law of physics and these are called physical quantities. Two supplementary units are also available apart from the basic 7 units. Check out the article provided below in order to know more specifications in the units and measurements chapter while preparing for the exam.
Need For Units and Measurements
If you are a physics student then there is a very essential need for the system and units to measure different quantities including length, area, and mass of a particular thing There are various aspects related to the measurement of a particular unit. When you are measuring various aspects related to a particular unit you need to consider the length, breadth, and also mass that it carries. There is an International System of units that is adopted by the world in order to calculate the standard measurements. The International System of units is widely accepted as a unit and measurement tool for developed countries.
Also Read: CBSE Class 12th Physics Syllabus 2022
Types of Units of Measurement
- Fundamental units are defined as fundamental quantities.
- Derived units are derived from the fundamental units for all other physical quantities.
Units and Measurements: Systems of Units
- FPS system is the system in which the unit of length is a foot, the unit of mass is a pound and the unit of time is a second.
- CGS system is the system in which the unit of length is centimeters, the unit of mass is gram and the unit of time is second.
- MKS system is the system in which the unit of length is meters, the unit of mass is kilogram and the unit of time is second.
- SI system is the system including these units:
Basic Units | ||
|---|---|---|
Quantity | Unit | Symbol of the unit |
Length | meter | m |
Mass | kilogram | kg |
Time | second | s |
Temperature | kelvin | K |
Electric current | ampere | A |
Number of particles | mole | mol |
Luminous intensity | candela | cd |
Supplementary Units | ||
Plane angle | radian | rad |
Solid angle | Steradian | sr |
Units and Measurements - Fundamental Units
- Meter is signified as M. It is the distance traveled by light in a vacuum during a time interval of (1/299792458) seconds.
- Kilogram is signified as KG and it is the mass of a platinum-iridium cylinder kept at the National Bureau of Weight and measurements, Paris.
- Second is signified as S and it is the time taken by the light of a specified wavelength emitted by a cesium-133 atom to execute 9192631770 vibrations.
- Ampere is signified as A and it is the current which when passed through two straight parallel conductors of infinite length and of negligible cross-section kept at a distance of 1 meter apart in the vacuum produces between them a force equal to 2 x 10-7 newton per meter length.
- Kelvin is signified as K and is the fraction 1/273.6 of the thermodynamic temperature of the triple point of water.
- Candela is signified as cd and it is defined as 1/60th of luminous intensity of 1 square centimeter of a perfect black body maintained at the freezing temperature of platinum (1773 0C).
- Mole is signified as MD and one mole is the amount of substance that contains elementary units equal to the number of atoms in 0.012 kg of carbon-12.
Units and Measurements - Derived Units
- Radian is signified as rad and it is the angle subtended at the center of the circle by the arc whose length is equal to the radius of the circle.
- Steradian is signified as Sr and it is the solid angle subtended at the center of a sphere by a spherical surface of an area equal to the square of its radius.
Significant Figures in Units and Measurements
Significant figures include the number of important single digits in the coefficient of expression in scientific notation. If there is a significant figure present in an expression then it will indicate the precision with which the engineer has indicated that specific quantity.
Units and Measurements- Significant Figures Rules
- All non-zero digits are significant.
- All zeroes between non-zero digits are significant.
- All zeroes to the right of the last non-zero digit are not significant in numbers without a decimal point.
- All zeroes to the right of a decimal point and to the left of a non-zero digit are not significant.
- All zeroes to the right of a decimal point and to the right of a non-zero digit are significant.
- In addition and subtraction, we should retain the least decimal place among the values operated, in the result.
- In multiplication and division, we should express the result with the least number of significant figures as associated with the least precise number in operation.
Units and Measurements- Significant Figures Examples
- 5688 – 4 significant figures
- 70.65 – 4 significant figures
- 680,000 – 2 significant figures
- 8.00 – 3 significant figures
- 0.00300 – 3 significant figures
Dimensions Of Physical Quantities
The dimension of a physical quantity tells us the fundamental units that have been used in order to measure that physical quantity.
Dimensional Analysis
The dimensional analysis will help us to know the relationship between physical quantities with the help of their dimensions and the units of measurement. It will also help us perform calculations within the same units, requiring conversion methods to be undertaken.
Application of Dimensional Analysis
- Dimensional analysis is used to check the consistency of an equation.
- The analysis will also help us to know the relation between physical quantities in physical phenomena
- Dimensional analysis is often used for conversion which is converting units from one system to another.
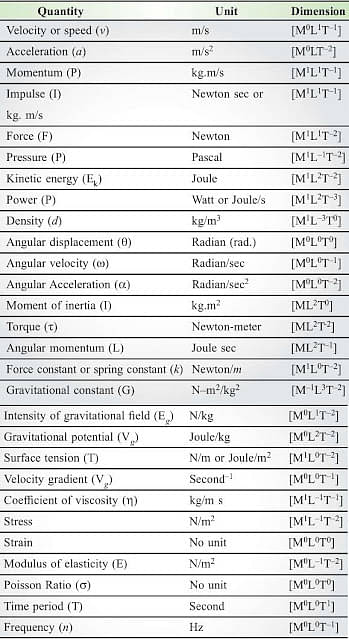
Units and Dimensions of Derived Quantities
Physical Quantity | Unit | Dimensional Formula |
|---|---|---|
Displacement | m | M0L1T0 |
Area | m2 | M0L2T0 |
Volume | m3 | M0L3T0 |
Velocity | ms-1 | M0L1T-1 |
Acceleration | ms-2 | M0L1T-2 |
Density | Kg m-3 | M1L-3T0 |
Momentum | Kg ms-1 | M1L1T-1 |
Work/Energy/Heat | Joule (or) Kg m2/sec2 | M1L2T-2 |
Power | Watt (W) (or) Joule/sec | M1L2T-3 |
Angular velocity | rad s-1 | M0L0T-1 |
Angular acceleration | rad s-2 | M0L0T-2 |
Moment of Inertia | Kg m2 | M1L2T0 |
Force | Newton (or) Kg m/sec2 | M1L1T-2 |
Pressure | Newton/m (or) Kg m-1/sec2 | M1L-1T-2 |
Impulse | Newton sec (or) Kg m/sec | M1L1T-1 |
Inertia | Kg m2 | M1L2T0 |
Electric Current | Ampere (or) C/sec | QT-1 |
Resistance/Impedance | Ohm (or) Kg m2/sec C2 | ML2T-1Q-2 |
EMF/Voltage/Potential | Volt (or) Kg m2/sec2 C | ML2T-2Q-1 |
Permeability | henry/m (or) Kg m/C2 | MLQ-2 |
Permittivity | Farad/m (or) sec2C2/Kgm3 | T2Q2M-1L-3 |
Frequency | Hertz (or) sec-1 | T-1 |
Wavelength | m | L1 |
Units and Measurements - Important Conversions Formulas
- 1 bar = 106 dyne/cm2 = 105 Nm-2 = 105 pascal
- 76 cm of Hg = 1.013×106 dyne/cm2 = 1.013×105 pascal = 1.013 bar.
- 1 toricelli or torr = 1 mm of Hg = 1.333×103 dyne/cm2 = 1.333 millibar.
- 1 kmph = 5/18 ms-1
- 1 dyne = 10-5 N,
- 1 H.P = 746 watt
- 1 kilowatt hour = 36×105 J
- 1 kgwt = g newton
- 1 calorie = 4.2 joule
- 1 electron volt = 1.602×10-19 joule
- 1 erg = 10-7 joule
Units and Measurements - Important Physical Constants
- Velocity of light in vacuum (c) = 3 × 108 ms-1
- Velocity of sound in air at STP = 331 ms-1
- Acceleration due to gravity (g) = 9.81 ms-2
- Avogadro number (N) = 6.023 × 1023/mol
- Density of water at 4oC = 1000 kgm-3 or 1 g/cc.
- Absolute zero = -273.15oC or 0 K
- Atomic mass unit = 1.66 × 10-27 kg
- Quantum of charge (e) = 1.602 × 10-19 C
- Stefan’s constant = 5.67 × 10–8 W/m2/K4
- Boltzmann’s constant (K) = 1.381 × 10-23 JK-1
- One atmosphere = 76 cm Hg = 1.013 × 105 Pa
- Mechanical equivalent of heat (J) = 4.186 J/cal
- Planck’s constant (h) = 6.626 × 10-34 Js
- Universal gas constant (R) = 8.314 J/mol–K
- Permeability of free space (μ0) = 4π × 10-7 Hm-1
- Permittivity of free space (ε0) = 8.854 × 10-12 Fm-1
- The density of air at S.T.P. = 1.293 kg m-3
- Universal gravitational constant = 6.67 × 10-11 Nm2kg-2
Units and Measurements - Some Important Questions With Solutions
Question - The density of a cube is calculated by measuring the length of one side and its mass. If the maximum errors in the measurement of mass and length are 3% and 2% respectively, then what is the maximum possible error in the measurement of density?
Answer: 3% + 3 × 2% = 9%
Question - A new unit of length is chosen such that the speed of light in a vacuum is unity. What is the distance between the Sun and the Earth in terms of the new unit if light takes 8 min and 20 s to cover this distance?
Answer: Distance between them = Speed of light x Time taken by light to cover the distance
Speed of light = 1 unit
Time taken = 8 x 60 + 20 = 480 + 20 = 500s
The distance between Sun and Earth = 1 x 500 = 500 units.
Also Read:
CBSE Board 12th Previous Year Question
The Units and Measurement chapter hold a large weightage for the physics students so it must be prepared accordingly. You can check out the specifications related to the important topics available in this subject from the article mentioned above.
Are you feeling lost and unsure about what career path to take after completing 12th standard?
Say goodbye to confusion and hello to a bright future!

Was this article helpful?




















Similar Articles
List of Documents Required for TS EDCET 2025 Counselling
Documents Required for Bihar B.Ed CET 2025 Counselling Process
Bihar B.Ed Round 1 Cutoff 2025
Bihar B.Ed CET 2025 Expected Cutoff: Check Previous Years' Cutoff Trends
Bihar B.Ed CET 2025 Cutoff: SC, ST, OBC, General, Good Score
TS TET 2025: Exam Dates (Jun 18-30), Admit Card (Jun 11), Registration Last Date (Apr 30), Eligibility, Syllabus & Latest Updates