 CBSE Class 12 Chemistry Daily Practice Questions 25 October 2023
CBSE Class 12 Chemistry Daily Practice Questions 25 October 2023CBSE Class 12 Chemistry Daily Practice Questions for 25 October 2023: Candidates are advised to solve CBSE Class 12th Chemistry practice questions daily to understand the concepts of chemistry better, It helps students to identify the areas where they need more practice and focus. Regular practice of CBSE Class 12 chemistry questions improves problem-solving skills, boosts their confidence, and prepares students for board exams effectively.
Also Check |
| CBSE Class 12 Chemistry Topic-Wise Weightage 2024 |
|---|
| CBSE Class 12 Chemistry Daily Practice Questions 20 October 2023 |
CBSE Class 12 Chemistry Daily Practice Questions for 25 October 2023
Students can analyse their CBSE Class 12 Chemistry exam performance by solving daily practice questions shared below.
Question 1: Multiple-Choice Questions
The ions of metals of Group 12 (Zn, Cd and Hg) have completely filled d orbitals and so they :
(a) behave like semiconductors
(b) are very high melting solids
(c) do not behave like transition metals
(d) behave like superconductors
Question 2: Very Short Answer Type Question
Name the cell which :
(a) was used in Apollo Space programme.
(b) is used in automobiles and inverters.
(c) is suitable for hearing aids and watches.
(d) does not give a steady potential and is used in transistors.
Question 3: Short Answer Type Question
Account for the following :
(a) Benzyl chloride is highly reactive towards S
N
1 reaction.
(b) (±)-Butan-2-ol is optically inactive, though it contains a chiral carbon atom.
(c) Chloroform is stored in closed dark coloured bottles.
Also Read |
CBSE likely to revise Class 10, 12 Exam Dates 2024 due to Lok Sabha and Assembly Elections
Question 4: Case-Based Question
Amines are usually formed from nitro compounds, halides, amides, imides, etc. They exhibit hydrogen bonding which influences their physical properties. In alkyl amines, a combination of electron releasing, steric and hydrogen bonding factors influence the stability of the substituted ammonium cations in protic polar solvents and thus affect the basic nature of amines. In aromatic amines, electron releasing and withdrawing groups, respectively increase and decrease their basic character. Influence of the number of hydrogen atoms at nitrogen atom on the type of reactions and nature of products is responsible for identification and distinction between primary, secondary and tertiary amines. Presence of amino group in aromatic ring enhances reactivity of the aromatic amines. Aryl diazonium salts provide advantageous methods for producing aryl halides, cyanides, phenols and arenes by reductive removal of the diazo group.
Answer the following questions :
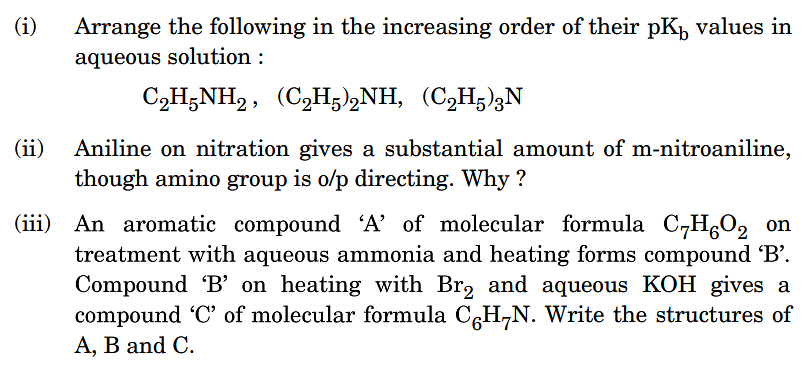
Question 5: Long Answer Type Question
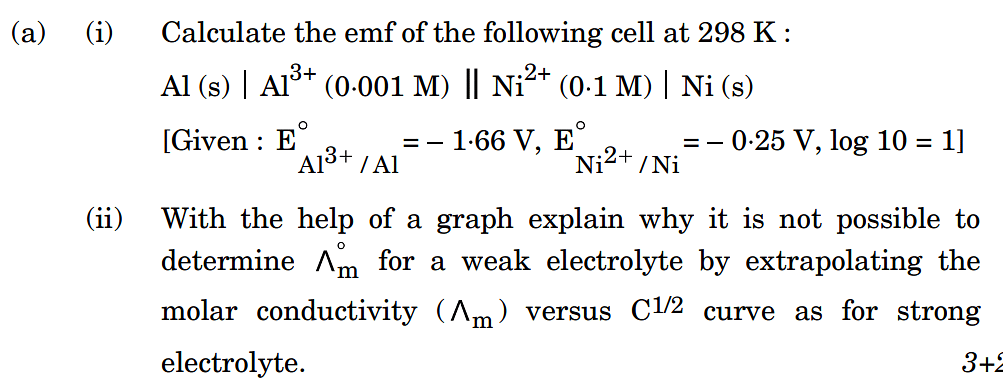
Also Read |
| CBSE Class 12 Physics Daily Practice Questions 25 October 2023 |
|---|
| CBSE Class 12 Mathematics Daily Practice Questions 25 October 2023 |
| CBSE Class 12 Biology Daily Practice Questions 25 October 2023 |
For the latest Education News , keep visiting CollegeDekho. You can also ‘follow’ our WhatsApp Channel to stay updated with the latest happenings. You can also write to us at our E-Mail ID news@collegedekho.com.
Are you feeling lost and unsure about what career path to take after completing 12th standard?
Say goodbye to confusion and hello to a bright future!

Was this article helpful?





 Follow us
Follow us












