- Introduction
- Discovery of Electron
- Discovery of Proton
- Discovery of Neutron
- Atomic Number, Isotopes, and Isobars
- Difference between Isotopes & Isobars
- Thomson's Model and Its Limitations
- Rutherford's Model and Its Limitations
- Bohr's Model and its Limitations
- Concept of Shells and Subshells
- Dual Nature of Matter and Light
- De Broglie's Relationship
- Heisenberg Uncertainty Principle
- Concept of Orbitals
- Quantum Numbers
- Shapes of s, p, and d Orbitals
- Rules for Filling Electrons in Orbitals - Aufbau Principle
- Electronic Configuration of Atoms
- Stability of Half-Filled and Completely-Filled Orbitals
- Structure of Atom: Previous Year’s Question Papers
Introduction
Atom is something that is present in everything we see around us at the most basic level. Every living or nonliving matter such as a table, water, animals, humans, etc. is made up of atoms. Without atoms, this world would not exist! So, we know that everything is made up of atoms but what are these atoms made up of? We will learn through this tutorial today!
Basically, Atoms have two main structure components:
- Atomic Nucleus consisting of Protons & Neutrons.
- Extra Nucleus consisting of Electrons.
Discovery of Electron
In 1897, a Physicist named JJ Thompson discovered that in every atom, there is at least one negative charged particle present. He named it initially “corpuscles” which was later named “electron.”
Hence, the Electron contributes to the negative charge of an atom and is denoted by ‘e’. The absolute charge of an electron is the negative charge of 1.6×10-19 coulombs. The relative mass of an electron is 1/1836. Furthermore, the mass of an electron is 0.
Discovery of Proton
In the early 1900s, a Physicist named Rutherford discovered the Proton while conducting the gold foil experiment. One of the scientists named Goldstein was conducting an experiment with the help of discharged tube using a perforated cathode. During the experiment, he found some rays which were positively charged. These rays were named canal rays and anode rays. Further, some more experiments lead to the discovery of Protons as a particle that consists of the positive charge of an atom.
Hence, the Proton contributes to the positive charge of an atom and is denoted by ‘p’. The absolute charge of a proton is the positive charge of 1.6×10-19 coulomb. The mass of a proton is 1.6×10 -24 g and is considered 1 which is equal to the mass of a hydrogen atom.
Discovery of Neutron
Neutron was discovered later in the year 1932 by Physicist James Chadwick. James calculated the mass of neutral particles using the scattered particles. A neutron is also known as a subatomic particle that is found in the nucleus of an atom.
Hence, the Neutron is a neutral particle that is neither positive nor negative and is denoted by ‘n’. The mass of a neutron is 1.6 x 10 -24 g. We cannot use Gram as a unit for calculating the tiny subatomic particles, hence, we use amu (atomic mass unit) or Dalton. The protons and neutrons have a mass that is nearly 1 amu.
The table below shows the characteristics of subatomic particles including their location, mass & charge:
Atomic Number, Isotopes, and Isobars
Atomic Number Definition
Atomic Number consists of an important characteristic of an atom. The definition of an Atomic Number says that:
The number of unit positive charges that is present in an atom nucleus for a specific element is known as the atomic number of that element.
But, we have just understood that the positive charge of an atom is Protons. So, we can rewrite the above definition as:
The number of Protons that is present in an atom nucleus for a specific element is known as the atomic number of that element.
In the case of a neutral atom, the number of protons is equal to the number of electrons. Therefore, we can say that the atomic number of an atom is equal to the number of electrons present in it. Based on this logic, we can write:
One more concept that we need to understand before moving further is the Atomic Mass Number. Atomic Mass Number is the sum of neutrons and protons present in the nucleus of an atom. It is denoted by A. Hence, we can write:
Isotopes
Isotopes are the atomic species of an element that has the same atomic numbers but different mass numbers. We can write the definition of Isotopes as:
The atom of an element having the same number of protons but a different number of neutrons is known as an isotope of that element.
Characteristics of Isotopes
We can see some of the characteristics of isotopes from the pointers provided below:
- The nuclei of Isotopes consist of the same number of protons but different numbers of neutrons. This is because they have the same atomic number but different atomic masses.
- The different isotopes of an element will have similar characteristics. This is because their chemical properties are determined by the number of protons and neutrons present.
Isobars
Isobars are the atomic species of different neighboring elements that have the same atomic mass numbers but different atomic numbers. We can write the definition of Isobars as:
The atom of two neighboring elements having the same number of neutrons but a different number of protons is known as isobars of these elements.
Characteristics of Isobars
We can see some of the characteristics of isobars from the pointers provided below:
- Since there are different atomic numbers, the isobars of both elements will have different numbers of neutrons and electrons.
- Moreover, as their mass numbers are the same, they will have a similar sum of neutrons and protons.
- Isobars will have different chemical properties.
- They are different atoms with the same mass number.
Difference between Isotopes & Isobars
The table below shows the major differences between Isotopes and Isobars:
Isotopes | Isobars |
|---|---|
They are atomic species of the same element. | They are atomic species of two different elements. |
They have the same atomic number but different atomic masses. | They have the same atomic masses but different atomic numbers. |
The number of electrons is the same. | The number of electrons is not the same. |
The number of protons is the same but the number of neutrons is different. | The number of protons and the number of neutrons is different. |
They have the same chemical properties. | They have different chemical properties. |
They have the same position on the periodic table. | They have different positions on the periodic table. |
Thomson's Model and Its Limitations
One of the major scientific models for an atom is Thomson’s Model of the Atom. In the year 1904, J.J. Thomson discovered electrons and proposed this model. Although, during that time, there was no discovery of an atomic nucleus. Hence, Thomson's model of the atom has been proposed on limited properties discovered until then. At that time, there were two properties of atoms known in Science:
- Electrons are the negatively charged particles that are present in an atom.
- Atoms are neutrally charged
Basic Theory of Thomson’s Model of Atom
As per the theory implemented by Thomson’s model of the Atom, the following points were represented:
- The atom constitutes a sphere where there are positive charges and negative charges are present inside the sphere.
- The magnitude of both positive and negative charges is equal. Hence, overall an atom is electrically neutral with no charge present as a whole.
- The model resembles the spherical structure of a watermelon. It resembles so because the electrons present in an atom look like seeds spread in the sphere of positive charge as the red part of the watermelon.
Limitations of Thomson’s Model of Atom
There are some limitations to Thomson’s Atomic Model which have been listed below:
- Thomson’s model of the atom failed to provide a logical explanation of how the electrons inside the atom are held by the positive charge.
- The model failed to mention anything regarding the nucleus of an atom.
- There were no explanations for the stability of an atom.
- The model was not able to explain Rutherford's scattering experiment.
Rutherford's Model and Its Limitations
As per Rutherford’s theory, an atom is made up of empty space where the electrons orbit in a set, there are positively charged nuclei and the paths are predictable and fixed.
Rutherford Model Experiment
Rutherford conducted a gold foil experiment where he transmitted high-energy α-particles on a thin gold foil of 100 nm thickness from a radioactive source. The experiment was conducted with the purpose to study the deflection that is caused in the path of α-particles when it comes in contact with the thin sheet of gold foil. To conduct this experiment, Rutherford placed a screen made of zinc sulfide around the gold foil. The observations gathered through the experiment contradicted Thomson’s Model of the Atom.
Rutherford Model Experiment Observations
The following observations were made by Rutherford’s gold foil experiment:
- Since most of the α-particles passed through the gold sheet without deflection, it was concluded that the majority of space in an atom is empty.
- Some of the α-particles were deflected by the gold foil sheet. However, the deflection was at a very small angle and minute. Hence, it was concluded that the protons (positive charge) in an atom are not uniformly distributed and are present in a very small volume.
- Very few of the α-particles deflected at large angles or in the opposite direction. Very few α-particles deflected at 180-degree angles. Hence, it was concluded that the protons (positive charge) are present in a very small volume as compared to the total volume of an atom.
Basic Theory of Rutherford’s Model of Atom
Based on the experiment and observations, the following theory has been presented by Rutherford’s Model of the Atom:
- The atom is made up of positively charged particles.
- Most of the mass of an atom is concentrated in a very small region known as the nucleus of an atom.
- The dense and small atom nuclei are made up of protons and neutrons.
- The nucleus of an atom is surrounded by electrons (negatively charged particles). These electrons move around the nucleus in a fixed path at a very high speed. These paths are also known as orbits.
- The nucleus of an atom is very small in size as compared to the total atom size.
- There is no net charge in an atom. That means they are electrically neutral because the nucleus is positively charged and the electrons are negatively charged.
- The electrons and nucleus are held together through a strong electrostatic force of attraction.
Limitations of Rutherford’s Model of Atom
There are some limitations to Rutherford’s Model of the Atom which have been listed below:
- Rutherford’s Model of the Atom failed to explain the atom’s stability like Thomson’s model.
- As per the model, the electrons move around the nucleus in a fixed path at a very high speed. This failed to adhere to Maxwell’s theory which states that accelerated charged particles release electromagnetic radiation.
- Rutherford’s Model did not explain anything about the electron's arrangement in orbit. This made the theory incomplete.
- Rutherford’s Model also failed to adhere to Maxwell’s mode as due to electrons' motions, the orbits will shrink gradually. This will make the orbit shrink and the nucleus of the atom will collapse. So, as per Maxwell’s model, Rutherford's model will collapse within 10-8 seconds.
Bohr's Model and its Limitations
After the failure of Thomson’s model and Rutherford’s model in terms of answering the questions related to the energy & stability of the atom, a new theory arose. In the year 1913, a Scientist named Neils Bohr proposed his own model of the atom. As per Bohr’s model, an atom is a positively charged, small nucleus surrounded by electrons that travel in a circular orbit due to the electrostatic force’s attraction. This is similar to the planets in our solar system which revolve around the sun due to its gravitational force.
Basically, Bohr’s model of the atom was an improved version of the previous two models which overcame their limitations. As per the structure proposed by Bohr, electrons move around a fixed orbit and not anywhere in between. The structure also places the theory that each orbit of electrons has a fixed energy level. It was also found that the electron that is located away from the nucleus tends to have more energy as compared to the electrons that are located closer to the nucleus.
Basic Theory of Bohr’s Model of Atom
The following theory has been presented by Bohr’s Model of the Atom:
- The electrons move around the nucleus in a fixed orbit and do not emit any energy. There is definite energy for each orbit which is called its energy level or energy shell.
- The orbit or energy level is denoted as K, L, M, and N shells. When the electron is in the lowest energy level, then it is said to be in the ground state.
- Whenever an electron moves from one energy level to another, it either emits or absorbs energy. For example, when it moves from a lower energy level to a higher energy level, it absorbs energy. Meanwhile, when it moved from a higher energy level to a lower energy level, it emits energy.
- The difference between two energy levels’ energies is equal to the energy absorbed or emitted. We can determine this using the plank’s equation:
ΔE = E2-E1 = hv
Where,
ΔE = energy absorbed or emitted
E2 and E1 = Energies
h= Plank’s constant
v= frequency of electromagnetic radiation emitted or absorbed
The angular momentum of an electron revolving in energy shells is given by:
mevr = nh2π
Where,
n= number of corresponding energy shell; 1, 2, 3 …..
me= mass of the electron
v= velocity
r=radius
h= Plank’s constant
Limitations of Bohr’s Model of Atom
There are some limitations to Bohr’s Model of the Atom which have been listed below:
- The model violates the Heisenberg Uncertainty Principle. As per Bohr’s model of the atom, the electrons have a fixed radius & orbit. This means it has a known position & momentum at the same time which is not possible as per the Heisenberg Uncertainty Principle.
- Even though the theory proposed by the model is correct for small size atoms like Hydrogen, it does not provide correct spectral predictions for large size atoms.
- The model failed to explain the Zeeman effect i.e. the splitting of spectral lines in the presence of a magnetic field.
- The model failed to explain the Stark effect i.e. the splitting of spectral lines in the presence of the electric field.
Concept of Shells and Subshells
As we know, electrons move constantly around the nucleus. These electrons move only within a specified path which is known as shell, subshell, and orbital. Even though both shells and subshells represent the path for electron movement, they differ in terms of energy & quantum number.

Concept of Shell
A shell refers to the path of an electron through which it moves around the nucleus of an atom. An atom consists of different shells of different energy levels around it. These shells are arranged in increasing order of energy levels around the nucleus. The shell having the least energy level will be in the first place and the shell with the highest energy level will be in last place. They are recognized as K, L, M, N, etc. Each shell can hold a number of electrons that are based on its principal quantum number (n). Its formula is 2n^2, where ‘n’ is the shell number.
Concept of Subshell
A subshell refers to the path of an electron through which it moves within a shell. They differ in terms of their energy and electron-holding capacity. The subshells are arranged in increasing order of energy levels around the nucleus. The subshells are named as per their angular momentum quantum number. Basically, there are 4 types of subshells: s, p, d, and f. The s subshell has the lowest energy followed by p, d, and f. They also have specific structures. The ‘s’ subshell has a spherical shape while the p has a dumbbell shape and the d has a double-dumbbell shape.
Dual Nature of Matter and Light
As per the theory of Albert Einstein in 1905, light has a dual nature. Einstein proposed the theory that lights act as a wave and as a particle. The energy quantum is associated with each particle and is known as a photon. This theory explains that when light is emitted through a prism, it splits into various bands of light at specific wavelengths and these bands are called line-emission spectrums for that specific element.
Dual Nature of Matter
The dual nature of matter was proposed by Lewis de-Broglie in the year 1924. The theory stated that matter has dual characteristics just like radiation. The following theories were presented in terms of the Dual Nature of Matter:
- The universe is made up of matter and electromagnetic radiation. Both of these are forms of energy that can be transformed into one another.
- Similar to the duality of radiation, matter also has a dual nature. This means that the matter has symmetry.
- As per Broglie’s concept, the moving matter composites have wave-like properties like interference & diffraction. While at the time of rest, it shows the properties of a particle. Hence, the matter has a dual nature.
- The waves which are associated with the moving particles are called de-Broglie waves or matter waves.
De Broglie's Relationship
To address the limitations of Bohr’s model of atom, some changes were made to rectify the model. One of these rectifications included the dual nature of matter. As per the nature of particles, the equation given by Einstein is written as,
E= mc2 …...(1)
Where,
E = energy
m = mass
c = speed of light
As per the nature of waves, we can write the Plank equation as,
E = hν …... (2)
Where,
E = energy
h = Plank’s constant
ν = frequency
Considering equation (1) and (2),
mc2 = hν …... (3)
Now, we can write frequency (ν) in terms of wavelength (λ) as
ν = c/λ
If we replace c with the velocity of the particle for a general particle, the equation (3) can be rewritten as,
mv2 = hv/λ ⇒ λ = h/mv
This above equation is known as the de Broglie relationship and the wavelength, λ is known as de Broglie wavelength.
Heisenberg Uncertainty Principle
As per Heisenberg Uncertainty Principle, there can be inherent uncertainty while measuring the variable of a particle. This principle is commonly applied to the momentum & position of a particle. As per the principle, the more precisely the position is known the more uncertain the momentum is, and vice versa. Heisenberg's Uncertainty Principle is one of the most important quantum mechanics theories that define why a scientist cannot measure multiple quantum variables simultaneously.
The principle states that the uncertainty in position/ momentum and time/ energy of the particle refers to the minimum value corresponding to Planck’s constant divided by 4π. Hence,
Δp Δx ≥ h/ 4π… (1)
Δt ΔE ≥ h/ 4π.. (2)
Where
Δ= The uncertainty in the variable and
h = Plank's constant.
The Heisenberg Uncertainty Principle implies so due to the wave-like nature of the particle. As we know, the particle in space is spread all over. Therefore, there cannot be a precise location of the particle rather, it occupies a range of positions. Similarly, the momentum cannot be precisely known since a particle consists of a packet of waves, each of which has its own momentum so at best it can be said that a particle has a range of momentum.
Figure: A wave packet in space
Concept of Orbitals
Orbital refers to a mathematical function that overviews the wave-like nature of an electron. The orbitals are also known as electron orbital or atomic orbital. Even though orbit is generally regarded as a circle, the probability density regions that may contain an electron may be spherical, dumbbell-shaped, or more complicated three-dimensional forms. The purpose of orbitals is to map the probability of the location of an electron in a region around (or theoretically inside) an atomic nucleus.
The orbital refers to an electron cloud where the energy levels are described by given values of the n, ℓ, and mℓ quantum numbers. For each electron, there is a unique quantum number set. In an orbital, there can be 2 electrons having paired spins, and is often associated with a specific region of an atom. The s orbital, p orbital, d orbital, and f orbital refer to orbitals that have an angular momentum quantum number ℓ = 0, 1, 2, and 3, respectively.
Quantum Numbers
4 quantum numbers are used to describe the movement of electrons in an atom. Each electron moving in an atom is described using a wave function that adheres to the Schrödinger equation. According to the Pauli Exclusion Principle, no two electrons can share the same combination of four quantum numbers. Quantum numbers are important as they are used to determine the configuration of an atom and the precise location of electrons in an atom. Quantum numbers are also used to understand the specific characteristics of atoms such as atomic radius, ionization energy, etc.
There are 4 quantum numbers used for atoms: the principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms). The principal quantum number, n, describes the energy of an electron and the most probable distance of the electron from the nucleus. In other words, it refers to the size of the orbital and the energy level at which an electron is placed. The number of subshells, or l, describes the shape of the orbital. It can also be used to determine the number of angular nodes. The magnetic quantum number, ml, describes the energy levels in a subshell, and ms refers to the spin on the electron, which can either be up or down.
Shapes of s, p, and d Orbitals
The shape of the orbitals for an atom varies due to the elimination of various energy-related contributions. The shape of different orbitals has been explained one by one in this section:
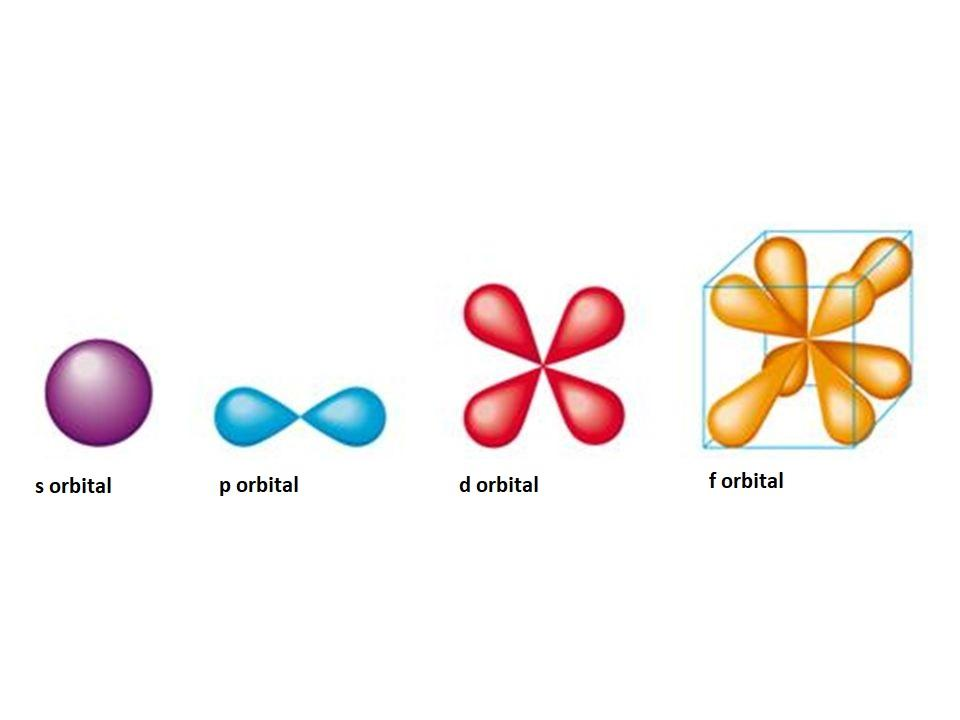
s-orbital Shape
- The s-orbital shape resembles a sphere where the nucleus is in the center. It can be shown in 2D as a circle.
- These orbitals are symmetrical spherically which means the electron at a given distance might be the same in all directions.
- With the increase in primary quantum number, the size of the s orbital will also increase; hence, 4s > 3s > 2s > 1s.
- The nodal point is a location where there is no chance of locating the electron.
p-Orbital Shape
- P orbitals shape resembles dumbbells.
- p orbital can occupy a maximum of six electrons as there are 3 orbitals present.
- The nodal point for p orbitals is located at the center of the nucleus.
- Each orbital is made up of lobes that are located on either side of the plane that runs across the nucleus.
- All the 3 orbitals have the same size, shape, and energy and are known as degenerate orbitals.
- With the increase in primary quantum number, the size of the s orbital will also increase hence, 4p > 3p > 2p.
d-Orbital Shape
- There are five d orbitals.
- The magnetic orbital quantum number for d orbitals are -2, -1, 0, 1, 2.
- These orbitals are denoted by the symbols dxy, dyz, dxz, dx2–y2, and dz2.
- The forms of the first four d orbitals are similar to each other, which differs from the dz2 orbital, but the energy of all five d orbitals is the same.
f-Orbital Shape
- The form of the f orbital is dispersed. Because the value of l=3 for the f orbital, the minimal value of the primary quantum number n is 4.
- The equivalent ml values for the f orbital are (-3,–2, –1, 0, +1, +2, +3).
- As a result, there are seven f orbitals for l = 3.
Rules for Filling Electrons in Orbitals - Aufbau Principle
The Aufbau Principle states that the electrons are filled into atomic orbitals in the sequence of increasing orbital energy levels. This principle is used to explain the placement of electrons in an atom along with their energy levels. For example, Carbon has 6 electrons and its electrical configuration is 1s22s22p2.
Some of the salient features of the Aufbau Principle are as follows:
- The principle states that electrons are placed at the lowest energy level orbitals first. Only when these orbitals are completely filled, do the electrons move to the higher energy-level orbitals.
- The sequence for the growth of the energy level of orbitals can be identified using the (n+l) rule, where the sum of the primary and azimuthal quantum numbers determines the energy level of the orbital.
- If the value of the above sum is low, it indicates that the orbital has a lower energy level and vice versa.
- Each and every orbital is filled with electrons in the order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Electronic Configuration of Atoms
The Electronic Configuration of Atoms is used to describe the arrangement of electrons in space around the nucleus. Based on the rules, the electron configuration of atoms of some elements is shown in the table below:
Element | Chemical Symbol | Atomic No. | Distribution of electrons over orbits (shells), 2n2 | Electronic configuration of atom | |||
|---|---|---|---|---|---|---|---|
K(n=1) | L(n=2) | M(n=3) | N(n=4) | ||||
Hydrogen | H | 1 | 1 | 0 | 0 | 0 | 1s1 |
Helium | He | 2 | 2 | 0 | 0 | 0 | 1s2 |
Lithium | Li | 3 | 2 | 1 | 0 | 0 | 1s2 2s1 |
Beryllium | Be | 4 | 2 | 2 | 0 | 0 | 1s2 2s2 |
Boron | B | 5 | 2 | 3 | 0 | 0 | 1s2 2s2 2p1 |
Carbon | C | 6 | 2 | 4 | 0 | 0 | 1s2 2s2 2p2 |
Nitrogen | N | 7 | 2 | 5 | 0 | 0 | 1s2 2s2 2p3 |
Oxygen | O | 8 | 2 | 6 | 0 | 0 | 1s2 2s2 2p4 |
Fluorine | F | 9 | 2 | 7 | 0 | 0 | 1s2 2s2 2p5 |
Neon | N | 10 | 2 | 8 | 0 | 0 | 1s2 2s2 2p6 |
Sodium | Na | 11 | 2 | 8 | 1 | 0 | 1s2 2s2 2p6 3s1 |
Magnesium | Mg | 12 | 2 | 8 | 2 | 0 | 1s2 2s2 2p6 3s2 |
Aluminum | Al | 13 | 2 | 8 | 3 | 0 | 1s2 2s2 2p6 3s2 3p1 |
Silicon | Si | 14 | 2 | 8 | 4 | 0 | 1s2 2s2 2p6 3s2 3p2 |
Phosphorus | P | 15 | 2 | 8 | 5 | 0 | 1s2 2s2 2p6 3s2 3p3 |
Sulphur | S | 16 | 2 | 8 | 6 | 0 | 1s2 2s2 2p6 3s2 3p4 |
Chlorine | Cl | 17 | 2 | 8 | 7 | 0 | 1s2 2s2 2p6 3s2 3p5 |
Argon | Ar | 18 | 2 | 8 | 8 | 0 | 1s2 2s2 2p6 3s2 3p6 |
Potassium | K | 19 | 2 | 8 | 8 | 1 | 1s2 2s2 2p6 3s2 3p6 4s1 Or [Ar]4s1 |
Calcium | Ca | 20 | 2 | 8 | 8 | 2 | 1s2 2s2 2p6 3s2 3p6 4s2 Or [Ar]4s2 |
Scandium | Sc | 21 | 2 | 8 | 8 | 2 | 1s2 2s2 2p6 3s2 3p6 3d1 4s2 Or [Ar]3d1 4s2 |
Where, [Ar] = 1s2 2s2 2p6 3s2 3p6 represents the electron configuration of Argon which is having completely filled 3-shells. It is used to simplify the electron configuration of elements of higher atomic numbers.
Stability of Half-Filled and Completely-Filled Orbitals
All the atoms and elements follow the same rules when writing electronic configurations. When the energies of two subshells differ, an electron from the lower energy subshell may travel to the higher energy subshell. This is because of the symmetrical distribution and exchange of energy between the electrons. Two or more electrons having the same spin can exchange their places with the degenerate orbitals. The spinning of electrons creates a new quantum mechanical interaction known as Exchange energy. Additionally, the exchange energy release is greatest in half-filled or filled subshells, boosting their stability. This is proportional to the number of exchanges of positive electrons by electrons with the same spins from one orbital to another in the same subshell.
Structure of Atom: Previous Year’s Question Papers
The students can also refer to the following previous years' questions to learn more about the type of questions asked in the exam:
Stay tuned to CollegeDekho for more Education News !
Are you feeling lost and unsure about what career path to take after completing 12th standard?
Say goodbye to confusion and hello to a bright future!

Was this article helpful?




















Similar Articles
RIE CEE 2025 Exam Day Guidelines
RIE CEE 2025 Login: Forgot Password, Steps to Retrieve
What is a Good Score in RIE CEE 2025?
Documents Required for Odisha B.Ed Entrance Exam 2025 Counselling Process
IIT Kharagpur ITEP Admission 2025: Admission Merit List (Out), Fees, Selection Process, Important Dates
IIT Bhubaneswar ITEP Admission 2025: Admission Merit List, Selection Process, Fees, Important Dates