Never Miss an Exam Update
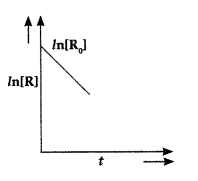
CBSE Class 12th Chemistry Chapter 3 - Chemical Kinetics includes the following topics: Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation. This chapter is included in the fourth unit of the syllabus. The weightage of the chapter will be 7 marks. The theory paper will be conducted for 70 marks. The theory paper will include different types of questions based on the curriculum followed by CBSE. There will be no overall choice in the question paper. However, 33% of internal choices will be given in all the sections.
The Central Board of Secondary Education has already released the latest curriculum on the official website of CBSE Academics. To prepare well for the exam, you must download the latest syllabus PDF from the official website and complete it as soon as possible. After completing your syllabus, you can move forward with the model test papers available on the official website and practice them one by one. After completing the sample question papers, you can also pick up the previous years' question papers uploaded on the official website of CBSE at cbse.nic.in. Since Chemistry is a practical subject, do not forget to also give importance to the practical exam. The practical exam will be conducted for 30 marks. The 30 marks will be divided into different parameters.
For Chemistry, you must make notes after the end of every chapter to revise efficiently. Keep your practical file up to date. Take part in group studies to clear your doubts easily. Take help from YouTube to understand difficult topics.
The Central Board of Secondary Education has already released the date sheet for class 12th, according to which the exams start on 15th February 2025. Given below, we are sharing the CBSE Class 12th Chemistry Chapter 3 - Chemical Kinetics Important Questions with Answers here. Make sure to attempt the questions after completing your syllabus, and you can also check the correct answers by clicking on the tab given below:
Was this article helpful?